HOME / daily use SPF / moisturizer with SPF

Vichy Laboratories Liftactiv Peptide C Sunscreen Lotion, SPF 30
Vichy Laboratories Liftactiv Peptide C Sunscreen Lotion, SPF 30

WHERE TO BUY
Health Concerns
-
LOWCancer
-
HIGHAllergies & Immunotoxicity
-
LOWDevelopmental and Reproductive Toxicity
-
HIGHUse Restrictions
Efficacy Concerns
-
Caution We have flagged this product with 2 concerns Sunscreens can break down while still in the bottle. To be safe dispose of products when the mixture clumps or separates.
This product contains chemical active ingredient(s) that the FDA does not have enough health safety data to classify as safe and effective: AVOBENZONE, HOMOSALATE, OCTISALATE, OCTOCRYLENE
-
someUVA/UVB Balance
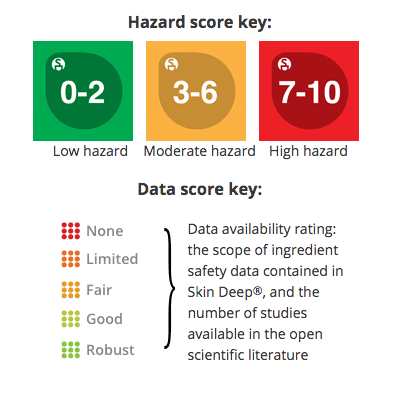
Ingredient Scores
Ingredients are scored based on their formulation and concentration in this product. Click on an ingredient for more information.

|
FRAGRANCE
Data Availability: Fair
|


|
||||
|
||||||

|
OCTISALATE active ingredient 5%
Data Availability: Fair
|


|
||||
|
||||||

|
HOMOSALATE active ingredient 5%
Data Availability: Fair
|


|
||||
|
||||||

|
DIMETHICONE
Data Availability: Limited
|


|
||||
|
||||||

|
GLYCOLIC ACID
Data Availability: Fair
|


|
||||
|
||||||

|
SODIUM HYDROXIDE
Data Availability: Fair
|


|
||||
|
||||||

|
PHENOXYETHANOL
Data Availability: Limited
|


|
||||
|
||||||

|
PEG-100 STEARATE
Data Availability: Limited
|


|
||||
|
||||||

|
STEARETH-100
Data Availability: Limited
|


|
||||
|
||||||

|
AVOBENZONE active ingredient 3%
Data Availability: Limited
|


|
||||
|
||||||

|
OCTOCRYLENE active ingredient 7%
Data Availability: Fair
|


|
||||
|
||||||

|
GLYCERIN
Data Availability: Good
|


|
||||
|
||||||

|
TOCOPHEROL
Data Availability: Fair
|


|
||||
|
||||||

|
TETRADIBUTYL PENTAERITHRITYL HYDROXYHYDROCINNAMATE
Data Availability: Limited
|


|
||||
|
||||||

|
WATER
Data Availability: Robust
|


|
||||
|
||||||

|
SILICA, AMORPHOUS
Data Availability: Good
|


|
||||
|
||||||

|
GLYCERYL MONOSTEARATE
Data Availability: Limited
|


|
||||
|
||||||

|
STEARIC ACID
Data Availability: Fair
|


|
||||
|
||||||

|
DICAPRYLYL CARBONATE
Data Availability: None
|


|
||||
|
||||||

|
DIMETHICONE/ VINYL DIMETHICONE CROSSPOLYMER
Data Availability: None
|


|
||||
|
||||||

|
SODIUM HYALURONATE
Data Availability: Fair
|


|
||||
|
||||||

|
MYRISTIC ACID
Data Availability: Fair
|


|
||||
|
||||||

|
CYCLODEXTRIN
Data Availability: Fair
|


|
||||
|
||||||

|
PALMITIC ACID
Data Availability: Fair
|


|
||||
|
||||||

|
PHENYLETHYL RESORCINOL
Data Availability: None
|


|
||||
|
||||||

|
GUANOSINE
Data Availability: Fair
|


|
||||
|
||||||

|
AMMONIUM POLYACRYLOYLDIMETHYL TAURATE
Data Availability: None
|


|
||||
|
||||||

|
ASCORBYL GLUCOSIDE
Data Availability: Limited
|


|
||||
|
||||||

|
CAPRYLYL GLYCOL
Data Availability: Limited
|


|
||||
|
||||||

|
SODIUM SALT ETHYLENEDIAMINE DISUCCINATE
Data Availability: Fair
|


|
||||
|
||||||

|
XANTHAN GUM
Data Availability: Fair
|


|
||||
|
||||||

|
PISUM SATIVUM (PEA) EXTRACT
Data Availability: Fair
|


|
||||
|
||||||
Ingredients from label
Active Ingredients: Avobenzone 3.0%, Homosalate 5.0%, Octisalate 5.0%, Octocrylene 7.0%; Inactive Ingredients: WATER, GLYCERIN, DIMETHICONE, SILICA, PEG-100 STEARATE, GLYCERYL STEARATE, GLYCOLIC ACID, STEARIC ACID, DICAPRYLYL CARBONATE, STEARETH-100, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, SODIUM HYDROXIDE, SODIUM HYALURONATE, MYRISTIC ACID, CYCLODEXTRIN, PALMITIC ACID, PHENOXYETHANOL, PHENYLETHYL RESORCINOL, GUANOSINE, AMMONIUM POLYACRYLOYLDIMETHYL TAURATE, ASCORBYL GLUCOSIDE, TOCOPHEROL, CAPRYLYL GLYCOL, TRISODIUM ETHYLENEDIAMINE DISUCCINATE, XANTHAN GUM, PENTAERYTHRITYL TETRA-DI-T-BUTYL HYDROXYHYDROCINNAMATE, PISUM SATIVUM (PEA) EXTRACT, FRAGRANCE
PETA: Companies That Do Test on Animals

People for the Ethical Treatment of Animals, a leading international animal rights advocacy organization, has identified companies that "either test on animals or pay a laboratory to conduct tests on animals."
LEARN MORE ON PETA.ORG