HOME / daily use SPF / moisturizer with SPF

ReVive Sensitif Oil Free Lotion, SPF 15 (2020 formulation)
ReVive Sensitif Oil Free Lotion, SPF 15 (2020 formulation)

WHERE TO BUY
Health Concerns
-
LOWCancer
-
MODERATEAllergies & Immunotoxicity
-
MODERATEDevelopmental and Reproductive Toxicity
-
HIGHUse Restrictions
Efficacy Concerns
-
Caution We have flagged this product with 2 concerns Sunscreens can break down while still in the bottle. To be safe dispose of products when the mixture clumps or separates.
This product contains chemical active ingredient(s) that the FDA does not have enough health safety data to classify as safe and effective: AVOBENZONE, OCTINOXATE
-
lowUVA/UVB Balance
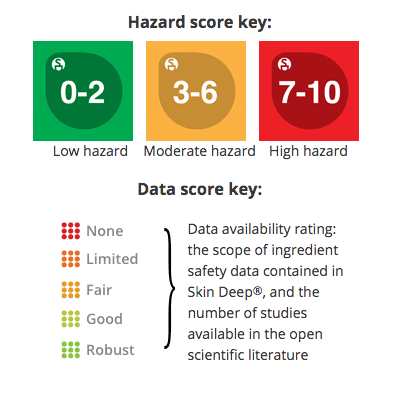
Ingredient Scores
Ingredients are scored based on their formulation and concentration in this product. Click on an ingredient for more information.

|
PROPYLPARABEN
Data Availability: Fair
|


|
||||
|
||||||

|
OCTINOXATE active ingredient 7.5%
Data Availability: Fair
|


|
||||
|
||||||

|
PHENOXYETHANOL
Data Availability: Limited
|


|
||||
|
||||||

|
AMINOMETHYL PROPANOL
Data Availability: Fair
|


|
||||
|
||||||

|
CITRUS AURANTIUM DULCIS (ORANGE) FRUIT EXTRACT
Data Availability: Limited
|


|
||||
|
||||||

|
METHYLPARABEN
Data Availability: Fair
|


|
||||
|
||||||

|
CYCLOPENTASILOXANE
Data Availability: Fair
|


|
||||
|
||||||

|
POLYSORBATE-60
Data Availability: Limited
|


|
||||
|
||||||

|
TOCOPHERYL ACETATE
Data Availability: Fair
|


|
||||
|
||||||

|
AVOBENZONE active ingredient 3%
Data Availability: Limited
|


|
||||
|
||||||

|
BISABOLOL
Data Availability: Limited
|


|
||||
|
||||||

|
WATER
Data Availability: Robust
|


|
||||
|
||||||

|
DIISOPROPYL SEBACATE
Data Availability: Limited
|


|
||||
|
||||||

|
BUTYLENE GLYCOL
Data Availability: Limited
|


|
||||
|
||||||

|
GLYCERYL MONOSTEARATE
Data Availability: Limited
|


|
||||
|
||||||

|
STEARIC ACID
Data Availability: Fair
|


|
||||
|
||||||

|
CETYL PHOSPHATE
Data Availability: Limited
|


|
||||
|
||||||

|
CETEARYL ALCOHOL
Data Availability: Limited
|


|
||||
|
||||||

|
SODIUM PCA
Data Availability: Limited
|


|
||||
|
||||||

|
ALLANTOIN
Data Availability: Fair
|


|
||||
|
||||||

|
CARBOMER
Data Availability: Fair
|


|
||||
|
||||||

|
DISODIUM EDTA
Data Availability: Fair
|


|
||||
|
||||||

|
LAMINARIA SACCHARINA EXTRACT
Data Availability: Limited
|


|
||||
|
||||||

|
GLYCOLIPIDS
Data Availability: Fair
|


|
||||
|
||||||

|
SODIUM HYALURONATE
Data Availability: Fair
|


|
||||
|
||||||

|
SH-OLIGOPEPTIDE-1
Data Availability: None
|


|
||||
|
||||||
Ingredients from label
Active Ingredients: Avobenzone 3.0%, Octinoxate 7.50%, Inactive Ingredients: Water, Diisopropyl Sebacate, Butylene Glycol, Cyclopentasiloxane, Glyceryl Stearate, Stearic Acid, Cetyl Phosphate, Cetearyl Alcohol, Phenoxyethanol, Sodium PCA, Polysorbate 60, Aminomethyl Propanol, Citrus Aurantium Dulcis (Orange) Fruit Extract, Allantoin, Methylparaben, Carbomer, Bisabolol, Propylparaben, Tocopheryl Acetate, Disodium EDTA, Laminaria Saccharina Extract, Glycolipids, Sodium Hyaluronate, sh-Oligopeptide-1
Unknown:
Leading international certifiers PETA and Leaping Bunny have no information concerning this company’s use of animal testing.