
Bottled water contains disinfection byproducts, fertilizer residue, and pain medication
The bottled water industry promotes an image of purity, but comprehensive testing by the Environmental Working Group (EWG) reveals a surprising array of chemical contaminants in every bottled water brand analyzed, including toxic byproducts of chlorination in Walmart’s Sam’s Choice and Giant Supermarket's Acadia brands, at levels no different than routinely found in tap water. Several Sam's Choice samples purchased in California exceeded legal limits for bottled water contaminants in that state. Cancer-causing contaminants in bottled water purchased in 5 states (North Carolina, California, Virginia, Delaware and Maryland) and the District of Columbia substantially exceeded the voluntary standards established by the bottled water industry.
Unlike tap water, where consumers are provided with test results every year, the bottled water industry is not required to disclose the results of any contaminant testing that it conducts. Instead, the industry hides behind the claim that bottled water is held to the same safety standards as tap water. But with promotional campaigns saturated with images of mountain springs, and prices 1,900 times the price of tap water, consumers are clearly led to believe that they are buying a product that has been purified to a level beyond the water that comes out of the garden hose.
To the contrary, our tests strongly indicate that the purity of bottled water cannot be trusted. Given the industry's refusal to make available data to support their claims of superiority, consumer confidence in the purity of bottled water is simply not justified.
Laboratory tests conducted for EWG at one of the country’s leading water quality laboratories found that 10 popular brands of bottled water, purchased from grocery stores and other retailers in 9 states and the District of Columbia, contained 38 chemical pollutants altogether, with an average of 8 contaminants in each brand. More than one-third of the chemicals found are not regulated in bottled water. In the Sam's Choice and Acadia brands levels of some chemicals exceeded legal limits in California as well as industry-sponsored voluntary safety standards. Four brands were also contaminated with bacteria.
Walmart and Giant Brands No Different than Tap Water
Two of 10 brands tested, Walmart's and Giant's store brands, bore the chemical signature of standard municipal water treatment — a cocktail of chlorine disinfection byproducts, and for Giant water, even fluoride. In other words, this bottled water was chemically indistinguishable from tap water. The only striking difference: the price tag. In both brands levels of disinfection byproducts exceeded safety standards established by the state of California and the bottled water industry:
- Walmart’s Sam’s Choice bottled water purchased at several locations in the San Francisco bay area was polluted with disinfection byproducts called trihalomethanes at levels that exceed the state’s legal limit for bottled water (CDPR 2008). These byproducts are linked to cancer and reproductive problems and form when disinfectants react with residual pollution in the water. Las Vegas tap water was the source for these bottles, according to Walmart representatives (EWG 2008).
- Also in Walmart’s Sam’s Choice brand, lab tests found a cancer-causing chemical called bromodichloromethane at levels that exceed safety standards for cancer-causing chemicals under California’s Safe Drinking Water and Toxic Enforcement Act of 1986 (Proposition 65, OEHHA 2008). EWG is filing suit under this act to ensure that Walmart posts a warning on bottles as required by law: “WARNING: This product contains a chemical known to the State of California to cause cancer."
- These same chemicals also polluted Giant's Acadia brand at levels in excess of California’s safety standards, but this brand is sold only in Mid-Atlantic states where California’s health-based limits do not apply. Nevertheless, disinfection byproducts in both Acadia and Sam’s Choice bottled water exceeded the industry trade association’s voluntary safety standards (IBWA 2008a), for samples purchased in Washington DC and 5 states (Delaware, Maryland, Virginia, North Carolina, and California). The bottled water industry boasts that its internal regulations are stricter than the FDA bottled water regulations(IBWA 2008b), but voluntary standards that companies are failing to meet are of little use in protecting public health.
Figure 1. Pollutants in Walmart and Giant Bottled Water Exceed Industry and California Standards 
The California legal limit of 10 parts per billion (ppb) for total trihalomethanes (TTHMs) in bottled water has been set by the California Health and Safety Code, Division 104, Part 5 (Sherman Food, Drug, and Cosmetic Law, CDPH 2008). The industry standard, Bottled Water Code of Practice, published by the International Bottled Water Association (IBWA 2008a), also sets a limit for TTHMs at 10 ppb. Two of the TTHM chemicals, bromodichloromethane and chloroform, are regulated in California under the Safe Drinking Water and Toxic Enforcement Act, also known as Proposition 65 (OEHHA 2008). For bromodichloromethane, a concentration above 2.5 ppb exceeds a cancer safety standard, as established by the state of California (OEHHA 2008). The standard is based on the Proposition 65 No Significant Risk Level for bromodichloromethane at 5 micrograms per day. For a water consumption rate of 2 L/day (Title 27, California Code of Regulations, Article 7, Section § 25721), this corresponds to a contaminant concentration in water of 2.5 ppb. The concentration values indicated by the bars correspond to findings from the specific brand purchased at the specific location. For the entire dataset, see section Walmart and Giant Water Exceeds Safety Limits. Two independent samples of Sam's Choice water were purchased in Oakland, CA, with total trihalomethane levels at 21 and 23 ppb and levels of bromodichloromethane at 7.7 and 8.5 ppb. Two independent samples of Acadia water were purchased in Stafford, VA with total trihalomethane levels at 22 and 23 ppb.
Broad Range of Pollutants Found in 10 Brands
Altogether, the analyses conducted by the University of Iowa Hygienic Laboratory of these 10 brands of bottled water revealed a wide range of pollutants, including not only disinfection byproducts, but also common urban wastewater pollutants like caffeine and pharmaceuticals (Tylenol); heavy metals and minerals including arsenic and radioactive isotopes; fertilizer residue (nitrate and ammonia); and a broad range of other, tentatively identified industrial chemicals used as solvents, plasticizers, viscosity decreasing agents, and propellants.
The identity of most brands in this study are anonymous. This is typical scientific practice for market-basket style testing programs. We consider these results to represent a snapshot of the market during the window of time in which we purchased samples. While our study findings show that consumers can't trust that bottled water is pure or cleaner than tap water, it was not designed to indicate pollutant profiles typical over time for particular brands. Walmart and Giant bottled water brands are named in this study because our first tests and numerous followup tests confirmed that these brands contained contaminants at levels that exceeded state standards or voluntary industry guidelines.
The study also included assays for breast cancer cell proliferation, conducted at the University of Missouri. One bottled water brand spurred a 78% increase in the growth of the breast cancer cells compared to the control sample, with 1,200 initial breast cancer cells multiplying to 32,000 in 4 days, versus only 18,000 for the control sample, indicating that chemical contaminants in the bottled water sample stimulated accelerated division of cancer cells. When estrogen-blocking chemicals were added, the effect was inhibited, showing that the cancer-spurring chemicals mimic estrogen, a hormone linked to breast cancer. Though this result is considered a modest effect relative to the potency of some other industrial chemicals in spurring breast cancer cell growth, the sheer volume of bottled water people consume elevates the health significance of the finding. While the specific chemical(s) responsible for this cancer cell proliferation were not identified in this pilot study, ingestion of endocrine-disrupting and cancer-promoting chemicals from plastics is considered to be a potentially important health concern (Le 2008).
With Bottled Water, You Don't Know What You're Getting
Americans drink twice as much bottled water today as they did ten years ago, for an annual total of over nine billion gallons with producer revenues nearing twelve billions (BMC 2007; IBWA 2008c). Purity should be included in a price that, at a typical cost of $3.79 per gallon, is 1,900 times the cost of public tap water.1 But EWG’s tests indicate that in some cases the industry may be delivering a beverage little cleaner than tap water, sold at a premium price. The health consequences of exposures to these complex mixtures of contaminants like those found in bottled water have never been studied.
Unlike public water utilities, bottled water companies are not required to notify their customers of the occurrence of contaminants in the water, or, in most states, to tell their customers where the water comes from, how and if it is purified, and if it is merely bottled tap water. Information provided on the U.S. EPA website clearly describes the lack of quality assurance for bottled water: "Bottled water is not necessarily safer than your tap water" (EPA 2007b). The Agency further adds following consumer information:
Some bottled water is treated more than tap water, while some is treated less or not treated at all. Bottled water costs much more than tap water on a per gallon basis... Consumers who choose to purchase bottled water should carefully read its label to understand what they are buying, whether it is a better taste, or a certain method of treatment (EPA 2007b).
In conjunction with this testing program, EWG conducted a survey of 228 brands of bottled water, compiling information from websites, labels and other marketing materials. We found that fewer than half describe the water source (i.e., municipal or natural) or provide any information on whether or how the water is treated. In the absence of complete disclosure on the label, consumers are left in the dark, making it difficult for shoppers to know if they are getting what they expect for the price.
Figure 2. Walmart and Giant Are Bottling Tap Water

The municipal water sources of the Walmart’s Sam’s Choice and Giant’s Acadia bottled waters were identified through contact with Walmart representatives, their bottled water manufacturer, and city/utility officials; or from the label (Giant). Data on the levels of disinfection byproducts (total trihalomethanes or TTHMs) in these municipal water sources were obtained from Notla Water Authority in Blairsville, Georgia; Las Vegas Valley Water District; and Washington Suburban Sanitary Commission. These data were from tap water tests carried out in 2007, which the water utilities disclosed to their customers in an annual report. For every utility the range of values from lowest to the highest represents the concentrations of TTHMs that were found in the tap water over the course of the year. Notla Water Authority provided a single value for TTHMs, not a range.
This study did not focus on the environmental impacts of bottled water, but they are striking and have been well publicized. Of the 36 billion bottles sold in 2006, only a fifth were recycled (Doss 2008). The rest ended up in landfills, incinerators, and as trash on land and in streams, rivers, and oceans. Water bottle production in the U.S. uses 1.5 million barrels of oil per every year, according to a U.S. Conference of Mayors’ resolution passed in 2007, enough energy to power 250,000 homes or fuel 100,000 cars for a year (US Mayors 2007). As oil prices are continuing to skyrocket, the direct and indirect costs of making and shipping and landfilling the water bottles continue to rise as well (Gashler 2008, Hauter 2008).
Extracting water for bottling places a strain on rivers, streams, and community drinking water supplies as well. When the water is not bottled from a municipal supply, companies instead draw it from groundwater supplies, rivers, springs or streams. This "water mining," as it is called, can remove substantial amounts of water that otherwise would have contributed to community water supplies or to the natural flow of streams and rivers (Boldt-Van Rooy 2003, Hyndman 2007, ECONorthwest, 2007).
Recommendations
Currently there is a double standard where tap water suppliers provide information to consumers on contaminants, filtration techniques, and source water; bottled water companies do not. This double standard must be eliminated immediately; Bottled water should conform to the same right-to-know standards as tap water. To bring bottled water up to the standards of tap water we recommend:
- Full disclosure of all test results for all contaminants. This must be done in a way that is readily available to the public.
- Disclosure of all treatment techniques used to purify the water, and:
- Clear and specific disclosure of the name and location of the source water.
To ensure that public health and the environment are protected, we recommend:
- Federal, state, and local policymakers must strengthen protections for rivers, streams, and groundwater that serve as America’s drinking water sources. Even though it is not necessarily any healthier, some Americans turn to bottled water in part because they distrust the quality of their tap water. And sometimes this is for good reason. Some drinking water (tap and bottled) is grossly polluted at its source – in rivers, streams, and underground aquifers fouled by decades of wastes that generations of political and business leaders have dismissed, ignored, and left for others to solve. A 2005 EWG study found nearly 300 contaminants in drinking water all across the country. Source water protection programs must be improved, implemented, and enforced nationwide (EWG 2005b). The environmental impacts associated with bottled water production and distribution aggravate the nation's water quality problems rather than contributing to their solution.
- Consumers should drink filtered tap water instead of bottled water. Americans pay an average of two-tenths of a cent per gallon to drink water from the tap. A carbon filter at the tap or in a pitcher costs a manageable $0.31 per gallon (12 times lower than the typical cost of bottled water), and removes many of the contaminants found in public tap water supplies.2 A whole-house carbon filter strips out chemicals not only from drinking water, but also from water used in the shower, clothes washer and dishwasher where they can volatilize into the air for families to breathe in. For an average four-person household, the cost for this system is about $0.25 per person per day.3 A single gallon of bottled water costs 15 times this amount.
EWG's study has revealed that bottled water can contain complex mixtures of industrial chemicals never tested for safety, and may be no cleaner than tap water. Given some bottled water company's failure to adhere to the industry's own purity standards, Americans cannot take the quality of bottled water for granted. Indeed, test results like those presented in this study may give many Americans reason enough to reconsider their habit of purchasing bottled water and turn back to the tap.
Footnotes.
1 A recent survey documented bottled water prices ranging from $0.89 to $8.26 per gallon (Food and Water Watch 2007). Retail prices vary widely depending on whether people are buying bottled water in bulk or individual bottles. Given this wide range in prices, EWG assumed a flat $1.00 per liter price per liter (or $3.79 per gallon), which is what most consumers would pay for a typical liter bottle of water bought from a convenience store. In comparison, EPA estimates that tap water costs consumers about $0.002 per gallon, on average, nationwide (EPA 2004).
2 EWG compared the prices and capacities of 7 faucet-mounted and pitcher filters. The prices ranged from $19.99 to $39.99 with treatment capacities ranging from 40 gallons to 100 gallons. With this information, we estimate an average cost of these types of systems as $0.31 per gallon.
3 EWG compared 5 different whole house carbon filter units and documented prices in the range between $64.99 to $795 per unit, with life spans between 3 and 36 months. Thus, the annual cost is in the range of $260 - $595 with an average of $375. This leads to an estimated cost of $1.00/day that translates into $0.25 daily cost per person for an average four-person household.
EWG’s Guide to Safe Drinking Water
 Drinking plenty of good, clean water is important for a healthy body. Read EWG researchers' top tips to learn how to stay hydrated while cutting down on your exposures to common drinking water pollutants.
Drinking plenty of good, clean water is important for a healthy body. Read EWG researchers' top tips to learn how to stay hydrated while cutting down on your exposures to common drinking water pollutants.
Bottled water
Drink filtered tap water instead. You can read the bottle label, but you still won't know if the water is pure, or just processed, polluted, packaged tap water. EWG found 38 contaminants in 10 popular brands.
Tap water
Learn what's in it. Tap water suppliers publish all their water quality tests. Bottled water companies don't. Read your annual tap water quality report. Look up your city's water in EWG's National Tap Water Atlas. (Private well? Get it tested.)
Filtered tap water
Drink it, cook with it.
- Choose a filter certified to remove contaminants found in your water: https://www.ewg.org/tapwater/water-filter-guide.php. Effectiveness varies - read the fine print.
- Carbon filters (pitcher or tap-mounted) are affordable and reduce many common water contaminants, like lead and byproducts of the disinfection process used to treat municipal tap water.
- If you can afford it, install a reverse osmosis filter to remove contaminants that carbon filters can't eliminate, such as chromium-6, arsenic and perchlorate (rocket fuel).
Filters
Change them. Change your water filters on time. Old filters aren't safe – they harbor bacteria and let contaminants through.
On the go
Carry water in safe containers. Hard plastic bottles (#7 plastic) can leach a harmful plastics chemical called bisphenol A (BPA) into water. Carry stainless steel or other BPA-free bottles. Don't reuse bottled water bottles. The plastic can harbor bacteria and break down to release plastics chemicals.
While Pregnant
Stay hydrated with safe water. It's especially important for women to drink plenty of water during pregnancy. Follow all the tips above, and take your doctor's advice on how much to drink.
Infants
Use safe water for formula. Use filtered tap water for your baby's formula. If your water is not fluoridated, you can use a carbon filter. If it is, use a reverse osmosis filter to remove the fluoride, because fluoridated water can damage an infant's developing teeth. If you choose bottled water for your infant, make sure it's fluoride-free.
Breathe Easy
Use a whole house water filter. For extra protection, a whole house carbon filter will remove contaminants from steamy vapors you and your family inhale while showering and washing dishes.
Walmart and Giant Water Exceeds Safety Limits
- Walmart’s Sam’s Choice bottled water purchased in the San Francisco bay area was polluted with disinfection byproducts called trihalomethanes at levels that violate the state’s legal limit for bottled water. These byproducts are linked to cancer and reproductive problems and form when disinfectants react with residual pollution in the water. The legal limit is 10 parts per billion (ppb) in bottled water in California (CDPR 2008); Walmart's bottled water purchased in Oakland and Mountain View contained more than double the limit (21 to 37 ppb). Las Vegas tap water was the source for these bottles, according to Walmart representatives (EWG 2008).
- Also in Walmart’s Sam’s Choice brand, lab tests found a cancer-causing chemical called bromodichloromethane at levels that exceed safety standards under California’s Safe Drinking Water and Toxic Enforcement Act of 1986 (Proposition 65, OEHHA 2008). EWG is filing suit under this act to ensure that Walmart posts a warning on bottles as required by law: “WARNING: This product contains a chemical known to the State of California to cause cancer." The limit for this chemical under Proposition 65 is 2.5 ppb, using the state's standard assumptions for water consumption; levels in Walmart's water from Mountain View and Oakland ranged from 7.7 to 13 ppb.
- These same chemicals also polluted Giant's Acadia brand at levels in excess of California’s safety standards, but this brand is sold only in Mid-Atlantic states where California’s health-based limits do not hold sway. Nevertheless, disinfection byproducts in both Acadia and Sam’s Choice bottled water exceeded the industry trade association’s voluntary safety standard (IBWA 2008) of 10 ppb for trihalomethanes, for samples purchased in 5 states and Washington DC. Acadia water with levels exceeding the industry's safety limit was purchased in 3 states (Maryland, Delaware and Virginia) and Washington, DC and was bottled from municipal tap water supplies in Maryland's DC suburbs, according to the bottle label. The Walmart water was purchased in California and North Carolina and was bottled from municipal tap water supplies in Las Vegas and Georgia, according to Walmart representatives (EWG 2008).
Most developed nations have guidelines to control disinfection byproducts in drinking water so as to minimize consumers' exposure to potentially hazardous chemicals while maintaining adequate disinfection and control of water-borne bacteria (Richardson 2007). EPA tap water regulations allow some quantities of these byproducts, which form when residual organic pollutants combine with chlorine and other water disinfection chemicals. Yet, largely unknown to consumers is the fact that FDA, the agency charged with overseeing bottled water quality, permits the same level of DBPs in bottled waters as allowed by the EPA for tap water (FDA 2008b). FDA-sanctioned presence of known carcinogens in bottled water highlights the woeful insufficiency of federal regulations over bottled water production. As a result of the FDA's hands-off approach to bottled water standards, quality among brands and even among different bottles within a single brand varies tremendously. As uncovered by EWG, while some bottled waters appear to be purified or treated more than tap water, others contain excessive levels of chemical pollutants.
EWG analysis of bottled waters sold by the Walmart and Giant Foods stores, discovered that every one of five Acadia brand waters and four out of eleven Sam's Choice brand waters contained disinfection byproducts, especially trihalomethanes (THMs), such as chloroform and bromodichloromethane, chemicals considered carcinogenic to humans (Richardson 2007) and included as such in the California's Proposition 65 list (OEHHA 2008). The trihalomethane levels detected in the nine samples are below the weak and nearly meaningless FDA limit of 80 parts per billion (ppb) for these chemicals in bottled water. However, all samples exceeded the bottled water industry self-proclaimed maximum level of 10 ppb for total THM contamination, with average trihalomethane levels of 25 ppb in Acadia's brand waters and 24 ppb in THM-containing Sam's Choice brand waters (Tables 1 and 2). These findings clearly demonstrate that in the absence of strong, enforceable federal standards, voluntary industry guidelines do not provide uniform bottled water quality promised to the consumers. Table 1. Acadia Filtered Drinking Water
| Purchase Location | Contaminants | Concentration Detected in Bottled Water |
| Middletown, DE | Chloroform | 25 ppb |
| Bromodichloromethane | 3.7 ppb | |
| Total Trihalomethanes | 29 ppb | |
| Fluoride | 0.91 ppm | |
| Silver Spring, MD | Chloroform | 12 ppb |
| Bromodichloromethane | 1.9 ppb | |
| Total Trihalomethanes | 14 ppb | |
| Fluoride | 0.76 ppm | |
| Stafford, VA (1) | Chloroform | 19 ppb |
| Bromodichloromethane | 2.7 ppb | |
| Total Trihalomethanes | 22 ppb | |
| Dichloroacetic Acid | 2 ppb | |
| Fluoride | 0.94 ppm | |
| Stafford, VA (2) | Chloroform | 20 ppb |
| Bromodichloromethane | 3 ppb | |
| Total Trihalomethanes | 23 ppb | |
| Fluoride | 0.87 ppm | |
| Washington, DC | Chloroform | 31 ppb |
| Bromodichloromethane | 4.9 ppb | |
| Total Trihalomethanes | 36 ppb | |
| Fluoride | 1.07 ppm |
Table 2. Sam's Choice Purified Drinking Water
| Purchase Location | Contaminants | Concentration Detected in Bottled Water |
| Mountain View, CA | Chloroform | 15 ppb |
| Bromodichloromethane | 13 ppb | |
| Chlorodibromomethane | 8.2 ppb | |
| Bromoform | 0.8 ppb | |
| Total Trihalomethanes | 37 ppb | |
| Oakland, CA (1) | Chloroform | 10 ppb |
| Bromodichloromethane | 8.5 ppb | |
| Chlorodibromomethane | 4.2 ppb | |
| Total Trihalomethanes | 23 ppb | |
| Oakland, CA (2) | Chloroform | 9.6 ppb |
| Bromodichloromethane | 7.7 ppb | |
| Chlorodibromomethane | 3.7 ppb | |
| Total Trihalomethanes | 21 ppb | |
| Fayetteville, NC | Chloroform | 12 ppb |
| Bromodichloromethane | 2.3 ppb | |
| Total Trihalomethanes | 14 ppb | |
| Camden, DE | Chloroform | ND* |
| Total Trihalomethanes | ND | |
| Cromwell, CT | Chloroform | ND |
| Total Trihalomethanes | ND | |
| Columbia, MD | Chloroform | ND |
| Total Trihalomethanes | ND | |
| Stafford, VA | Chloroform | ND |
| Total Trihalomethanes | ND | |
| Portland, OR | Chloroform | ND |
| Total Trihalomethanes | ND | |
| Vancouver, WA | Chloroform | ND |
| Total Trihalomethanes | ND | |
| Los Angeles, CA | Chloroform | ND |
| Total Trihalomethanes | ND |
*ND (not detected): samples did not contain these chemicals above detection limits.
In addition to being more than twice higher than the voluntary standard to which the bottled water industry clearly fails to adhere, the detected THM levels exceeded the health-protective limit of 10 ppb set for THMs in bottled water by the state of California (CDPH 2008). EWG testing raised especial concerns about Sam's Choice brand water retailed in California. Among the four tested Sam's Choice bottled waters from California stores, three contained trihalomethanes, and all three were over the 10 ppb CA state limit, with average concentration of 27 ppb.
The mixture of trihalomethanes in California-retailed Sam's Choice waters included chloroform, a known human carcinogen (NTP 2005) regulated in California under the Safe Drinking Water and Toxic Enforcement Act of 1986 (Proposition 65). According to the California EPA Office of Environmental Health Hazard Assessment (OEHHA), a safety standard for oral exposure to chloroform is at 10 ppb concentration (OEHHA 2008). The standard is based on the Proposition 65 No Significant Risk Level for ingested chloroform at 20 micrograms per day. For a water consumption rate of 2 L/day (Title 27, California Code of Regulations, Article 7, Section § 25721), this corresponds to a 10 ppb concentration of contaminant in drinking water. The levels of chloroform detected in three out of four Sam's Choice bottled waters from CA are between 9.6 and 15 ppb, very close to or over this limit. And while this level of exposure may be tolerated by a healthy person with average daily water consumption, it could pose greater risks for persons who consume significantly larger quantities of water every day or for vulnerable subpopulations.
In addition to chloroform, two other trihalomethanes were detected in Sam's Choice waters purchased in California: bromodichloromethane (average concentration 9.7 ppb) and chlorodibromomethane (average concentration 5.3 ppb). Acadia's brand contained bromodichloromethane at 3.2 ppb average concentration. Both bromodichloromethane and chlorodibromomethane are genotoxic and carcinogenic in animal studies (Richardson 2007). Like chloroform, bromodichloromethane is listed in the California's Safe Drinking Water and Toxic Enforcement Act (OEHHA 2008), with the safety standard of 5 micrograms per day, corresponding to 2.5 ppb concentration in water. The concentration of bromodichloromethane in three California samples of Sam's Choice water exceeded this guideline between three and five times, potentially posing an unacceptable risk to bottled water drinkers.
Why are disinfection byproducts tainting bottled water?
The bottled water industry builds its sales marketing the image of purity and casting doubt on the quality of tap water, leading bottled water drinkers to believe that they are purchasing a pristine product with no health risks whatsoever (Doss 2008, Edberg 2008). Less touted by the industry is the fact that bottled water manufacturers can and do use ordinary municipal tap water supplies to fill up the bottles (FDA 2008b). After the water has been pumped from the source and treated at taxpayers' expense, bottled water companies sell it back to the consumers at a vastly increased cost. As uncovered by the EWG investigation, some bottled waters contain signature tap water pollutants, essentially defeating consumers' purpose of seeking better water quality.
Under FDA regulations, bottled waters are legally allowed to contain the same quantities and types of chemical contaminants as public water supplies (FDA2008b). These lax rules for contaminants in bottled water benefit the most those bottled water suppliers who unscrupulously use taxpayer-supported tap water supplies to make their products. While FDA requires source labeling for bottled water drawn from municipal water supplies, manufacturers can avoid mandated disclosure by claiming to use additional purification (21 CFR 165.110(a)(3); FDA 2008b). To illustrate, the label on the Sam's Choice Purified Drinking Water purchased in Oakland, CA does not mention the source of water, instead describing the product as "Purified by reverse osmosis filtration or distillation." Nevertheless, this sample contained 10 ppb chloroform, 8.5 ppb bromodichloromethane, and 23 ppb total trihalomethanes, all in excess of California state standards (CDPH 2008).
Customer service representatives from Walmart provided EWG researchers with the locations of each municipal water supply used to fill the bottles EWG tested, matching the printed code number on each bottle to their supplier list. This was accomplished through a series of phone calls between EWG researchers and representatives on the companies' 1-888 numbers.
EWG investigation of the sources of four THM-containing Sam's Choice waters indicated that in every case, levels of THMs in the bottled water were close to levels of THMs in the local municipal water (Table 3). The safety of consumers would have been much better served if the FDA mandated a complete and unambiguous label disclosure whenever a bottled water has been sourced from tap water. Such transparent labeling would give the consumers the information to decide whether or not a certain bottled water best suits their needs.
Table 3. Comparison of Sam's Choice Purified Drinking Water with Local Source Tap Water
| Purchase Location | Contaminants | Concentration Detected in Bottled Water | Level (Range) Detected in Municipal Water (the Bottled Water Source) in 2007 |
| Mountain View, CA | Total Trihalomethanes | 37 ppb | 51.1 (7.8- 88) ppb1 |
| Fluoride | ND | 0.78 (0.38- 0.86) ppm | |
| Oakland, CA (1) | Total Trihalomethanes | 23 ppb | 51.1 (7.8- 88)1 |
| Fluoride | ND | 0.78 (0.38- 0.86) | |
| Oakland, CA (2) | Total Trihalomethanes | 21 ppb | 51.1 (7.8- 88)1 |
| Fluoride | ND | 0.78 (0.38- 0.86) | |
| Fayetteville, NC | Total Trihalomethanes | 14 ppb | 8 ppb (range not available)2 |
| Fluoride | ND | 1.6 (0.2-1.60 ppm) |
1 Las Vegas Valley Water District 2008
2 Blairsville, GA - Notla Water Authority 2008
As demonstrated by the EWG test results, when FDA-approved drinking water purification technologies are conscientiously applied, complete elimination of trihalomethanes can be achieved. Of the eleven tested samples of Sam's Choice Purified Drinking Water, seven of them did not contain any trihalomethanes. These included Sam's Choice waters purchased in Connecticut, Washington, Oregon, Delaware, Maryland, and Virginia and in the city of Los Angeles. In contrast, four of the waters from the same brand - those purchased in Fayetteville, North Carolina, and the cities of Mountain View and Oakland in California - contained trihalomethanes at levels that exceeded the industry's voluntary limit, the State of California standard for bottled water and "no significant risk levels" for carcinogens under Proposition 65. Such a disparity between different bottles from the same brand likely stems from non-uniform application of purification technologies by the bottlers at different sites, indicating that brand loyalty may not guarantee the bottled water quality that consumers seek.
EWG also examined the labeling of the Giant Food's Acadia brand of Filtered Drinking Water. This brand discloses on its label the public water source from which the bottled water was prepared and the treatment method applied (filtration through activated charcoal). While the Acadia labeling is in compliance with the letter of the law, it fails to alert the consumers that the bottled water contains levels of chloroform and other trihalomethanes that are above the industry's voluntary standard of 10 ppb. Overall, the levels of trihalomethanes and fluoride in the five tested samples of Acadia water were very close to the levels in the local source water (Table 4).
Table 4. Comparison of Acadia Filtered Drinking Water with Local Source Tap Water
| Purchase Location | Contaminants | Concentration Detected in Bottled Water | Level (Range) Detected in Source Water in 20071 |
| Middletown, DE | Total Trihalomethanes | 29 ppb | 43.8 (8.44-113) ppb |
| Fluoride | 0.91 ppm | 1.04 (0.52-1.40) ppm, 0.91 (0.10-1.10) ppm |
|
| Silver Spring, MD | Total Trihalomethanes | 14 ppb | 43.8 (8.44-113) ppb |
| Fluoride | 0.76 ppm | 1.04 (0.52-1.40) ppm, 0.91 (0.10-1.10) ppm | |
| Stafford, VA (1) | Total Trihalomethanes | 22 ppb | 43.8 (8.44-113) ppb |
| Fluoride | 0.94 ppm | 1.04 (0.52-1.40) ppm, 0.91 (0.10-1.10) ppm |
|
| Stafford, VA (2) | Total Trihalomethanes | 23 ppb | 43.8 (8.44-113) ppb |
| Fluoride | 0.87 ppm | 1.04 (0.52-1.40) ppm, 0.91 (0.10-1.10) ppm |
|
| Washington, DC | Total Trihalomethanes | 36 ppb | 43.8 (8.44-113) ppb |
| Fluoride | 1.07 ppm | 1.04 (0.52-1.40) ppm, 0.91 (0.10-1.10) ppm |
1 Washington Suburban Sanitary Commission 2008
In summary, the presence of disinfection byproducts in bottled waters highlights insufficient government oversight and inappropriate labeling of the bottled water products. As a result of the hands-off attitude of the FDA and cost-saving shortcuts taken by the industry itself, shoppers remain in a "Buyer Beware" situation, paying premium prices for bottled water but not getting the anticipated quality. Consumers could have obtained much better drinking water simply by installing a home tap water filter at a fraction of the bottled water cost. Consumers' right to know, market fairness, and individual shoppers' health are all affected by the sales of bottled waters that are no better than tap water - and vastly more expensive.
Test Results: Chemicals in Bottled Water
Chemical contaminants in drinking water pose a health risk for all of us, although some people may be more vulnerable to these pollutants than the general population. These more sensitive populations include infants, the elderly, as well as people with weakened immune systems due to viral infections, immune disorders, cancer, chemotherapy or recent organ transplants (CDPH 2008; EPA 2005a). Concerned about tap water quality, some consumers turn to bottled water, hoping to find a guarantee of safety and quality (Doss 2008; IBWA 2008d). But the reality is very different from this expectation: all bottled waters tested by EWG contained some chemical contaminants while bottled waters sold by two national retailers contained signature pollutants at levels very close to water.
Water treatment chemicals: disinfection byproducts and fluoride
 Toxic disinfection byproducts (DBPs) such as chloroform, bromodichloromethane, and haloacetic acids, are formed when disinfectants (chlorine, ozone, chlorine dioxide or chloramine) react with organic matter, urban and agricultural contaminants, bromine, and iodide during the treatment of drinking water (EPA 2008a). While only eleven DBPs are currently regulated in the U.S., up to 600 different chemicals may form as byproducts of disinfection (Richardson 1998, 1999a,b, 2003), including 74 DBPs that are not regulated but that may be associated with either DNA damage or carcinogenicity (Richardson 2007). In 2002, EWG review of DBP health effects found that nearly thirty peer-reviewed epidemiologic studies linked these byproducts to increased risks of cancer, including up to 9,300 cases of bladder cancer (reviewed in EWG 2002). DBP exposure may be also associated with miscarriages or reduced birth weight, a public health risk that is under active investigation (Hoffman 2008; Savitz 2006; Wright 2004). Additional health problems from DBP exposure may include rectal and colon cancers, kidney and spleen disorders, immune system problems and neurotoxic effects (EPA 2001a; EPA 2007a; Richardson 2007).
Toxic disinfection byproducts (DBPs) such as chloroform, bromodichloromethane, and haloacetic acids, are formed when disinfectants (chlorine, ozone, chlorine dioxide or chloramine) react with organic matter, urban and agricultural contaminants, bromine, and iodide during the treatment of drinking water (EPA 2008a). While only eleven DBPs are currently regulated in the U.S., up to 600 different chemicals may form as byproducts of disinfection (Richardson 1998, 1999a,b, 2003), including 74 DBPs that are not regulated but that may be associated with either DNA damage or carcinogenicity (Richardson 2007). In 2002, EWG review of DBP health effects found that nearly thirty peer-reviewed epidemiologic studies linked these byproducts to increased risks of cancer, including up to 9,300 cases of bladder cancer (reviewed in EWG 2002). DBP exposure may be also associated with miscarriages or reduced birth weight, a public health risk that is under active investigation (Hoffman 2008; Savitz 2006; Wright 2004). Additional health problems from DBP exposure may include rectal and colon cancers, kidney and spleen disorders, immune system problems and neurotoxic effects (EPA 2001a; EPA 2007a; Richardson 2007).
Trihalomethanes — Four chemicals found in EWG bottled water tests are in a group of disinfection byproducts called trihalomethanes (THMs) - chloroform, bromoform, bromodichloromethane, and chlorodibromomethane. Together, these chemicals can be present at the same 80 ppb concentration in bottled water as the EPA limit for THMs in tap water (EPA 2008b; FDA 2008b). The legal limit of 80 ppb was set as a compromise between protecting public health and the treatment costs for lowering THM levels in municipal water (EPA 2007a). This limit still equates to several thousands of bladder cancer cases nationwide from people ingesting THMs in drinking water (EPA 2001a; EPA 2005b). Various trihalomethanes were detected in four brands of bottled water, including Sam's Choice and Acadia, at two to three times greater levels than the bottled water industry's voluntary standard of 10 ppb (IBWA 2008).
During the first round of testing, chloroform was found in four brands at concentrations between 3.8 and 19 ppb. The second round of testing identified samples with up to 31 ppb concentration of chloroform. Among all THM-containing bottled waters in this study, average concentrations of 15 ppb chloroform were detected. Both the International Agency for Research on Cancer (IARC) and the U.S. National Toxicology Panel (NTP) state that chloroform is "reasonably anticipated to be a human carcinogen" (NTP 2005). Chloroform is listed as a carcinogen in the California's Safe Drinking Water and Toxic Enforcement Act (also known as Proposition 65), with safety standards for oral ingestion at 10 ppb (OEHHA 2008). The primary routes of human exposure to chloroform are ingestion, inhalation, and dermal contact with water while showering, swimming, cleaning, and cooking, so that practically all humans are exposed to low levels of the chemical (NTP 2005). Moreover, EPA was forced by a court order to weaken its health-based goal for chloroform from 0 ppb to 70 ppb as a result of a legal challenge filed by the Chlorine Chemistry Council and the Chemical Manufacturers Association (now the American Chemistry Council) (EPA 2008c).
Bromodichloromethane was detected in four brands and the total of eleven samples at concentrations between 0.6 and 13 ppb, with average of detected values at 4.5 ppb. EPA's Integrated Risk Information System (IRIS) classifies bromodichloromethane as a probable human carcinogen (EPA 1993) and EPA set a health-based goal (Maximum Contaminant Level Goal) for this cancer-causing chemical at zero (EPA 2008b). California's Safe Drinking Water and Toxic Enforcement Act lists 2.5 ppb as a safety standard for bromodichloromethane, a level that is exceeded by several fold for nine of the eleven THM-containing bottled waters. Two other THMs, chlorodibromomethane and bromoform, were found in Sam's Choice brand water, of which three samples contained chlorodibromomethane at concentrations between 3.7 and 8.2 ppb.
Haloacetic acids - Our tests found two water disinfection byproducts called haloacetic acids in bottled water, dichloroacetic acid and trichloroacetic acid, both at 2 ppb concentration. Haloacetic acids are genotoxic and carcinogenic; they can also produce significant metabolic disturbances (Robertson 2007). Both EPA and the International Agency for Research on Cancer consider dichloroacetic acid likely to be a carcinogen in humans (EPA 2003). While the available toxicity data for tricloroacetic acid is more limited, EPA IRIS assessment for this chemical reports cancer effects in rodents and classifies it as a possible human carcinogen (EPA 1996). Haloacetic acids are also linked to developmental defects in embryos grown outside the womb (whole embryo cell culture) (Hunter 1996). Prior to 2002, haloacetic acids were not regulated in drinking water at all. Now they are regulated as a group of five acids with a cumulative legal limit of 60 ppb in drinking water, whether tap or bottled (EPA 2008b; FDA 2008b). Similar to regulation of THMs in drinking water, the standard for haloacetic acid is not a health-based limit. Instead, it balances health and treatment cost by placing a dollar amount of the disease and equating that to treatment costs, so it still allows illness (EPA 2007a).
Disinfection byproducts were found in 4 brands
| Chemical | Number of Brands |
Range of Detections, ppb* | Average of Detected Values, ppb* | |
| Total Trihalomethanes | 4 | 4.4 - 37 | 21 | |
| Chloroform | 4 | 3.8 - 31 | 15 | |
| Bromodichloromethane | 4 | 0.6-13 | 4.5 | |
| Bromoform | 1 | 0.8 | 0.8 | |
| Chlorodibromomethane | 1 | 3.7 - 8.2 | 5.4 | |
| Haloacetic Acids | ||||
| Dichloroacetic acid | 2 | 2 | 2 | |
| Trichloroacetic acid | 1 | 2 | 2 | |
*ppb = parts per billion (micrograms per liter)
Fluoride was found in five brands at concentrations between 0.15 and 1.07 ppm (parts per million, same as mg/L). Fluoride in bottled water may be coming from natural sources or, for the bottled water brands that use tap water, fluoride may originate from municipal water treatment (FDA 2008b). The value of fluoride-containing toothpaste to dental health is clear; fluoride is a potent chemical that strengthens teeth and kills microbes on contact, reducing the incidence of cavities (Hellwig 2004; ten Cate 1999; Twetman 2003). But, as recently reviewed by the National Research Council (NRC) a substantial and growing body of peer-reviewed science strongly suggests that ingesting fluoride in drinking water may present serious health risks (NRC 2006). Children who drink fluoridated water are at increased risk of developing fluorosis, a defect of the permanent teeth resulting in dark staining and, in severe cases, substantial corrosion of the enamel (Hong 2006; McDonagh 2000; NRC 2006). The Center for Disease Control (CDC) stated that about 30% of children who drink fluoridated water have some degree of fluorosis (Beltran-Aguilar 2005).
Levels of fluoride now detected in bottled water, 0.15-1.07 ppm, are within legal limits (EPA 1989, FDA 2008b), but emerging science suggests that legal limits may not sufficiently protect health, especially for infants and others who are particularly vulnerable (NRC 2006).
Fluoride was found in 5 brands
| Chemical | Number of Brands |
Range of Detections, ppm* | Average of Detected Values, ppm* |
| Fluoride | 5 | 0.15-1.07 | 0.67 |
*ppm = parts per million (milligrams per liter, mg/L)
Fertilizer pollution: Nitrate and Ammonia
 Nitrate — Nitrate is a fertilizer ingredient that widely pollutes drinking water sources nationwide. It poses particular risks for infants, who are susceptible to a form of methemoglobinemia, or blue-baby syndrome, caused by nitrate replacing the oxygen normally carried by red blood cells (Knobeloch 2000). For babies and small children, the most common source of nitrate exposure is from infant formula, when it is mixed with well water (Kross 1992).
Nitrate — Nitrate is a fertilizer ingredient that widely pollutes drinking water sources nationwide. It poses particular risks for infants, who are susceptible to a form of methemoglobinemia, or blue-baby syndrome, caused by nitrate replacing the oxygen normally carried by red blood cells (Knobeloch 2000). For babies and small children, the most common source of nitrate exposure is from infant formula, when it is mixed with well water (Kross 1992).
Nitrate was found in six brands, at concentrations between 0.1 - 1.7 ppm, with average level (among the six positive brands) of 0.5 ppm. Although nitrate levels detected in bottled water are below the legal limit of 10 ppm, this limit provides no margin of safety for infants. According to EPA, infants below the age of six months who drink water containing nitrate in excess of the drinking water standard could become seriously ill and, if untreated, may die (EPA 2001b). Moreover, studies of infants in Europe have found that three to four percent of methemoglobinemia cases in infants occur at even lower levels, below the legal limit (Sattelmacher 1964; Simon 1962). Additionally, exposure to nitrates in drinking water for pregnant women has been linked to possible adverse reproductive and developmental effects (Manassaram 2006). While the spectrum of nitrate-associated adverse health outcomes remains a subject of active research, a 2006 review by the Centers for Disease Control and Prevention (CDC) scientists summarized nine different epidemiologic studies conducted between 1982 and 2004 that observed nervous system defects, miscarriage, premature birth, impaired growth of babies in utero, and various birth defects linked to higher nitrate levels in drinking water (Manassaram 2006).
Nitrate pollution is also associated with potential endocrine-disrupting effects. Emerging science suggests that nitrate derived from agricultural run-off is capable of disrupting the functioning of thyroid hormones and reproductive hormones, thus contributing to the overall environmental load of endocrine disrupting chemicals to which humans and animals are exposed (Edwards 2006; Guillette & Edwards 2005; Guillette 2006; Hotchkiss 2008; McDaniel 2008).
Ammonia — One bottled water brand contained ammonia at 0.12 ppm concentration. Ammonia enters water from fertilizer runoff, leaching septic tanks, and erosion of natural deposits. It is also commonly found in household cleaners. Whether present as an ingredient in cleaners or as a pollutant in tap water, ammonia volatilizes into the air; people are exposed primarily by breathing it in. Ammonia triggers asthma attacks in some people and at high levels of exposure it is linked to a broader range of health problems (Makarovsky 2008). According to a 2004 government review: "We do not know if exposure to ammonia causes birth defects, or if it can pass to the fetus across the placenta or to infants via breast milk" (ATSDR 2004).
Fertilizer Pollution was found in 6 brands
| Chemical | Number of Brands |
Range of Detections, ppm* | Average of Detected Values, ppm* | |
| Nitrate (Nitrogen as N) | 6 | 0.1 - 1.7 | 0.51 | |
| Ammonia (Nitrogen as N) | 1 | 0.12 | 0.12 | |
*ppm = parts per million (milligrams per liter, mg/L)
Drugs
 Over the past two years, investigations all around the country found a variety of pharmaceutical residues in streams, lakes, and in drinking water (Kolpin 2002; EPA 2008d). Pharmaceuticals routinely taken by people are not fully absorbed by our bodies, and are excreted and passed first into wastewater and then into surface water. Similarly, medical waste and disposal of unused pharmaceuticals down the drain can add to the load of pharmaceuticals in surface waters (EPA 2008e). Drugs in the environment pose grave ecological risks; they also end up in our drinking water supplies (Hawthorne 2008; Mendoza 2008). EPA has yet to determine what risks to human health may be posed by pharmaceuticals in drinking water, especially for vulnerable subpopulations such as fetuses, infants, and those with weakened immune system (Daughton 2004). Meanwhile, these potential risks cannot be currently dismissed.
Over the past two years, investigations all around the country found a variety of pharmaceutical residues in streams, lakes, and in drinking water (Kolpin 2002; EPA 2008d). Pharmaceuticals routinely taken by people are not fully absorbed by our bodies, and are excreted and passed first into wastewater and then into surface water. Similarly, medical waste and disposal of unused pharmaceuticals down the drain can add to the load of pharmaceuticals in surface waters (EPA 2008e). Drugs in the environment pose grave ecological risks; they also end up in our drinking water supplies (Hawthorne 2008; Mendoza 2008). EPA has yet to determine what risks to human health may be posed by pharmaceuticals in drinking water, especially for vulnerable subpopulations such as fetuses, infants, and those with weakened immune system (Daughton 2004). Meanwhile, these potential risks cannot be currently dismissed.
Acetaminophen - Shoppers concerned about pharmaceuticals in tap water may consider turning to bottled water as a supposedly safer alternative. However, EWG analysis detected acetaminophen (Tylenol) in two bottled water brands at levels similar to what has been found in tap water in Chicago and Philadelphia (AP 2008; Hawthorne 2008). The concentrations in bottled water are below the average therapeutic dosage; however, effects of life-long, constant exposure to this levels of acetaminophen are not known.
Caffeine pollution of rivers and streams has become so wide-spread that U.S. Geological Survey and Department of Agriculture researchers consider it to be a key indicator for water contaminated by urban waste (Focazio 2008; Moore 2008). An article on the FDA website describes consumer perception that bottled water contains no caffeine, no calories and no sugar (Bullers 2002). And while the last two claims are generally true, the first one is not - EWG testing revealed an unexpected presence of caffeine residues in bottled water. The caffeine levels detected in bottled water are very close to those found in untreated sources of drinking water and in tap water (Focazio 2008; Grumbles 2008; Hawthorne 2008). Although these levels pose no health concern, being many times below what is found in a cup of coffee or a can of soda (Grumbles 2008), they do indicate likely exposure of the bottled water source to urban wastewater and various other contaminants associated with it.
Drugs and drug breakdown products were found in 3 brands
| Chemical | Number of Brands |
Range of Detections, ppt* | Average of Detected Values, ppt* | |
| Acetaminophen | 2 | 1.1 - 1.3 | 1.2 | |
| Caffeine | 1 | 51 | 51 | |
| 1,7-Dimethylxanthine (breakdown product of caffeine) |
1 | 10 | 10 | |
*ppt = parts per trillion (nanograms per liter)
Synthetic chemicals used in chemical industry and in plastic production: Acetaldehyde, Isobutane, nonanoic acid, toluene
 Nine brands contained plastic/industrial synthetic chemicals, for a total of twenty-two chemicals, between one and four detections for each. Ten chemicals were detected once, four were detected twice, five chemicals were present in three brands (2-methyl-1-propene, 3-methyl pentane, isobutane, methylcyclopentane, octane) and hexane, toluene and acetaldehyde were present in four brands each.
Nine brands contained plastic/industrial synthetic chemicals, for a total of twenty-two chemicals, between one and four detections for each. Ten chemicals were detected once, four were detected twice, five chemicals were present in three brands (2-methyl-1-propene, 3-methyl pentane, isobutane, methylcyclopentane, octane) and hexane, toluene and acetaldehyde were present in four brands each.
How do plastic/industrial synthetic chemicals end up in bottled water? From the moment of production at the manufacturing plant and until the time of consumption, bottled water is exposed to a wide variety of plastic chemicals that leach from packaging. The main type of packaging for bottled water is polyethylene terephthalate or PET, identified by recycling code 1. Besides the PET polymer, plastic packaging for bottled water also contains a variety of additives, catalyst chemicals that are involved in plastic synthesis process, chemicals that impart physical stability and resistance to packaging, sunscreen chemicals that protect the bottle from discoloration caused by exposure to UV light, and odor-scavenger substances that eliminate the smells associated with chemicals leaching from plastic. The FDA Inventory of Effective Food Contact Substance Notifications lists 23 different chemical products or mixes that may be legally added to PET plastics for bottled water packaging (FDA 2008d). Upon long-term storage, some of these chemicals could potentially leach from the plastic into the bottled water itself.
Acetaldehyde is one of the most common contaminants released from PET bottles during overheating or any type of thermal degradation (Cwiek-Ludwicka 2003; Darowska 2003; Eberhartinger 1990; Monarca 1994; Nawrocki 2002). EWG testing detected acetaldehyde in four bottled water brands, in the range of 0.6 - 36 ppb. Inhaled acetaldehyde poses a risk for genetic mutations and cancer, and it is classified by the EPA IRIS as a probable human carcinogen (EPA 1991). Acetaldehyde ingestion causes adverse health effects ranging from irritation of the digestive tract to liver damage (NAS 1995). Despite these health concerns, FDA has not established a legal limit for acetaldehyde in bottled water.
Hexane, another industrial chemical for which no drinking water standards have been established, was found in four brands. Nationwide tap water analyses conducted by EWG showed that 69 public water suppliers in four states were contaminated with hexane (EWG 2005b). Hexane has been associated with potential health impacts including developmental toxicity, neurotoxicity, reproductive toxicity, respiratory toxicity, and skin sensitivity (EPA 2005c).
Toluene was detected in four brands. Toluene is a petroleum-derived industrial chemical and a solvent for paints, paint thinners, silicone sealants, rubber, printing ink, adhesives (glues), lacquers, leather tanners, and disinfectants (ATSDR 2000). As a result of its extensive use, toluene contaminates water supplies nationwide, so that 31.8 million people in 1,009 communities drank water contaminated with toluene (EWG 2005b). The presence of toluene in drinking water presents a significant public health risk, since health impacts associated with toluene include cardiovascular or blood toxicity, developmental toxicity, gastrointestinal or liver toxicity, immunotoxicity, kidney toxicity, neurotoxicity, reproductive toxicity, respiratory toxicity, and skin sensitivity (EPA 2005d). EPA established a limit for toluene in drinking water at 1 ppm (mg/L), which was adopted by the FDA as a standard for bottled water (FDA 2008b). Although the toluene levels detected in our study were significantly lower than the legal limit, they highlight the issue that surface and ground water nationwide has been contaminated with industrial chemicals. The only reliable, long-term solution to water quality problems is cleaning up our water supplies and making sure that drinking water sources are protected from chemical pollution.
Synthetic chemicals were found in 9 brands
| Chemical | Number of Brands |
Range of Detections, ppb* | Average of Detected Values, ppb* | |
| Acetaldehyde | 4 | 0.6 - 36 | 9.7 | |
| Hexane | 4 | 0.2 - 0.8 | 0.55 | |
| Toluene | 4 | 0.5 - 2.9 | 1.5 | |
| 2-Methyl-1-propene | 3 | 0.3 - 0.6 | 0.47 | |
| 3-Methyl pentane | 3 | 0.3 - 0.8 | 0.47 | |
| Isobutane | 3 | 2.3 - 13.3 | 7 | |
| Methylcyclopentane | 3 | 0.7 - 1.3 | 0.9 | |
| Octane | 3 | 0.2 - 4 | 1.7 | |
| 3-Methyl heptane | 2 | 0.4 - 0.6 | 0.5 | |
| Cyclohexane | 2 | 0.4 - 1.3 | 0.73 | |
| Decane | 2 | 0.6 - 1.5 | 0.93 | |
| Heptadecane | 2 | 0.3 - 1.2 | 0.75 | |
| (Z)-13-Docosenamide | 1 | 1.2 | 1.2 | |
| 1-Hexene | 1 | 0.2 | 0.2 | |
| Hexadecanamide | 1 | 0.7 | 0.7 | |
| Hexadecane | 1 | 0.5 | 0.5 | |
| Methyl cyclopentane | 1 | 1.3 | 1.3 | |
| Naphthalene | 1 | 0.3 | 0.3 | |
| Nonadecane | 1 | 0.4 | 0.4 | |
| Nonanoic acid | 1 | 0.4 | 0.4 | |
| o-Hydroxybiphenyl | 1 | 1.0 | 1.0 | |
| Tetrachloroethene | 1 | 0.5 | 0.5 | |
* ppb = parts per billion (micrograms per liter)
Bacterial contamination
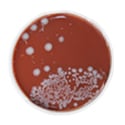 Four brands had some bacterial contamination, as detected by either total coliform count or heterotrophic plate count (HPC). One brand had particularly high background bacterial levels measured by HPC at 480 Colony-Forming Units (CFU) per milliliter, almost at the EPA's recommended limit of 500 CFU/ml for tap water (EPA 2008c). Although the presence of bacteria detected by the HPC method does not give a direct indication of potential risk for water-borne diseases, it is a measure of overall bacterial contamination that occurs during bottle water production. High HPC signal could indicate unsanitary conditions at the bottled water plant or bottled water collection site, possibly associated with dirty equipment. According to EPA, "the lower the concentration of bacteria in drinking water, the better maintained the water system is" (EPA 2008c).
Four brands had some bacterial contamination, as detected by either total coliform count or heterotrophic plate count (HPC). One brand had particularly high background bacterial levels measured by HPC at 480 Colony-Forming Units (CFU) per milliliter, almost at the EPA's recommended limit of 500 CFU/ml for tap water (EPA 2008c). Although the presence of bacteria detected by the HPC method does not give a direct indication of potential risk for water-borne diseases, it is a measure of overall bacterial contamination that occurs during bottle water production. High HPC signal could indicate unsanitary conditions at the bottled water plant or bottled water collection site, possibly associated with dirty equipment. According to EPA, "the lower the concentration of bacteria in drinking water, the better maintained the water system is" (EPA 2008c).
In addition to heterotrophic plate count, one brand was also positive for total coliform, which could indicate potential exposure of the bottled water sources to fecal contamination (FDA 2008c). While ground water is believed to contain less microbiological pollution compared to surface water, with the increased anthropogenic pressure on the environment, ground water frequently becomes tainted with bacteria from wastewater (EPA 2006). Potential sources of subsurface fecal contamination include improperly stored or managed manure from concentrated animal feeding operations (factory farms), runoff from land-applied manure, leaking sewer lines or failed septic systems, as well as entry of surface contaminants into the well due to improper construction or maintenance. FDA has recently proposed a new set of rules for improved monitoring of bacterial contamination in the sources used for bottled water production (FDA 2008c). However, these new rules would merely bring bottled water regulations in line with the EPA tap water regulations, so that standards for microbiological safety of bottled water would be at least no worse than tap water standards. And currently, all consumers can hope for is voluntary monitoring by the bottled water industry itself.
Bacterial contamination was found in 4 brands
| Bacterial Type | Number of Brands |
Range of Detections | Average of Detected Values | |
| Heterotrophic Plate Count | 4 | 1-480 CFU*/mL | 121 CFU/mL | |
| Total Coliform | 1 | 1 MPN**/100mL | 1 MPN/100mL | |
*CFU, colony-forming units; **MPN, most probable number of microorganisms.
Arsenic
Arsenic was found in one brand, at 1 ppb concentration. Arsenic is a metal that enters water by erosion of natural deposits, as well as industrial runoff. Inorganic arsenic has potent pesticide properties and is very toxic to people upon ingestion or inhalation. Potential health impacts associated with arsenic include cancer, cardiovascular or blood toxicity, developmental toxicity, endocrine toxicity, gastrointestinal or liver toxicity, kidney toxicity, neurotoxicity, reproductive toxicity, respiratory toxicity, and skin sensitivity (EPA 1998). In 2005, EWG investigation revealed that 90 million Americans in 38 states were served tap water contaminated with arsenic at levels above health-based limits between 1998 and 2003 (EWG 2005b). The FDA bottled water regulations allow the presence of arsenic up to 10 ppb concentration (FDA 2008b). However, considering that arsenic is a known human carcinogen, bottled water companies should ensure that their products be completely free from this dangerous pollutant. Nevertheless, the voluntary bottled water industry code allows arsenic contamination at 10 ppb levels (IBWA 2008a), a far cry from the industry claim to have internal guidelines that are more strict than the federal regulations.
Radioactive pollutants

Radioactivity — Gross beta particle radioactivity was detected in seven brands with average level of 3.7 pCi/L (picoCuries/liter). In humans and animals exposure to radioactivity causes a wide range of health effects, including lung, bone, liver, kidney and brain tumors, leukemia, skin damage, and blood damage. Two specific radiological contaminants were detected in bottled waters tested, Radium-228 and Strontium-90, and both are known cancer-causing elements. Radium-228 occurs naturally and is usually found around uranium deposits, while Strontium-90 is a radioactive pollutant from nuclear fallout and possibly weapons and power production. FDA regulations for radiological contaminants in bottled water allow the presence of gross beta radioactivity at levels not to exceed 4 millirems per year of human exposure (equivalent to 50 pCi/L (IBWA 2008a)) and the presence of Radium (Radium 226 and 228 combined) up to 5 pCi/L (FDA 2008b). While radiological contaminants detected in bottled water are below this legal limit, there is no level of radioactivity known to be without risk.
Radioactivity contamination was found in 7 brands
| Radioactivity Type | Number of Brands |
Range of Detections, pCi/L* | Average of Detected Values, pCi/L | |
| Gross Beta | 7 | 1.8-5.8 | 3.7 | |
| Radium-228 | 1 | 0.6 +/- 0.7 | 0.6 +/- 0.7 | |
| Strontium-90 | 1 | 0.5 +/- 0.4 | 0.5 +/- 0.4 | |
*pCi/L = picoCuries/liter
Boron
Boron was found in two brands, at 60 and 90 ppb (microgram/L) concentrations. Boron gets into drinking water from naturally-occurring and human sources. Contamination of water can come directly from urban and industrial wastewater and indirectly from soil runoff. People are exposed to this element with both water and food, since boron can be naturally found in some plants. Boron typically combines with oxygen to form various boron compounds that can contaminate drinking water. Boron is an unregulated chemical with no limits established by the EPA, although the World Health Organization, noting potential link between discharge of municipal sewage effluent and boron contamination, published a provisional guideline value for boron at 0.5 mg/L (WHO 2003). In animal studies, ingestion of boron has been linked with toxicity to male reproductive tract (testicular lesions) and developmental toxicity (WHO 2003). Boron has been listed in drinking water Contaminant Candidate Lists 1 and 2, which is a list of priority contaminants for which drinking water standards are urgently needed (EPA 2008f). For a decade, EPA vacillated on issuing tap water regulations for boron (EPA 2008f), even though the Agency acknowledges that lifetime ingestion of boron and boron compounds can increase the health effect risk for the fetuses of pregnant women and the testes of males (EPA 2008g). While the boron concentration found in this study are below the WHO levels, our finding highlights that adequate purification methods are not applied to water before bottling.
Conclusion: bottled water tainted with a mix of chemical pollutants from different sources
EWG investigation found chemical contamination in all bottled waters tested. The quality of the samples varied significantly, with some bottled waters exposing consumers to unexpectedly high pollution load. EWG study highlighted that weak FDA regulations are unable to ensure bottled water quality that consumers expect. Bottled water is not a miracle product - it is subjected to the same environmental contamination pressures as tap water. In the information provided by the EPA,
Whether it travels through a pipe to your home or comes packaged in a bottle... all our drinking water comes from similar sources, either from sources we can see, such as rivers and lakes, or from sources we can't see, such as underground aquifers (EPA 2005a).
Bottled water is not an answer to the search for drinking water free of chemical pollutants. Instead, protection of source water quality and better tap water treatment strategies are urgently needed to ensure that all Americans will continue to have access to safe and healthy water.
Is FDA Able to Ensure Bottled Water Quality?
Under FDA's bottled water regulations, bottled water is not required to be any safer than tap water. In fact, the chemical pollution standards are identical, with the sole exception of lead, for which FDA limits are stricter than the EPA limits (FDA 2008b; FDA 2002). Moreover, the current microbiological standards are weaker for bottled water compared to tap water (FDA 2008c).
When it comes to bottled water, FDA largely takes a hands-off approach. As stated on the FDA website, "bottled water plants generally are assigned low priority for inspection" (FDA 2002). Moreover, firms that use a public water system for their bottled water production may rely on public water system testing results instead of conducting their own independent testing, while other bottlers may reduce the frequency of their testing, as well as the number of chemical contaminants for which they test by obtaining a state-issued waiver (Title 21 CFR 129.35(a)(4)(i-ii)). As a result of weak standards and insufficient oversight, bottled water can be contaminated with various chemical and bacterial pollutants. Unfairly, consumers are left in the dark about these quality problems, since, unlike the municipal water companies, bottled water companies are not required to make public their water testing results. And many drinking water contaminants are unregulated - any level is legal.
Current FDA regulation of microbiological contaminants in bottled water is particularly embarrassing; the standards do not even specify which microorganisms should be tested or what levels of source water contamination will make it unfit for bottling (Title 21 CFR 129.35(a)(3)(i)). Finished bottled water products must be tested for total coliform; however, FDA allows up to 9.2 coliform organisms in 100 ml of bottled water (21 CFR 165.110(b)(2)). Recently, FDA proposed a rule to make microbiological quality standards for bottled water sources as strict as the EPA standards for tap water (FDA 2008c). Although it would serve as a much needed step to protect public health, the new rule would not guarantee that bottled water is safer than tap water. Instead, the only enforcement mechanism would be a requirement that a bottled water drawn from contaminated sources or tainted with microbiological contaminants carry a label with a statement of substandard quality. According to FDA: "A statement of substandard quality only prevents bottled water that exceeds an allowable level for a contaminant from being misbranded... it does not prevent the water from being adulterated" (FDA 2008c). Given the history of inappropriate labeling and lack of full disclosure by the bottled water industry, this rule does not seem sufficient to guarantee bottled water quality for consumers.
How can consumers know whether they are purchasing a reliable product or paying up a premium for over-priced tap water packaged in a questionable plastic bottle? Under Title 21 of the Code of Federal Regulations, bottlers are obligated to list on the label the type of bottled water and, for bottled water sourced from a public water system, the label must disclose that fact (21 CFR 165.110(a)(3)). However, this requirement can be circumvented by the bottlers. Simply by using water that has been "purified", "deionized" or "distilled", bottlers are free from legal obligation to disclose the tap water origin of their product (FDA 2008b). As a result, our health is left at risk - and manufacturers who wish to cut corners and neglect appropriate treatment of water before bottling can easily do so.
Voluntary industry standards claim to be more protective than the FDA regulations (Doss 2008; IBWA 2008b). However, precisely since these standards are voluntary, there is no monitoring or enforcement mechanism in place. As a result, many bottled waters tested by EWG contained levels of disinfection by-products more than twice higher than the industry self-proclaimed voluntary standard. Voluntary compliance or, more frequently, lack of such, cannot substitute for appropriate government regulations that will protect the health of people and the environment.
In summary, FDA needs to close the loophole that allows bottlers to avoid disclosing municipal sources of their waters. FDA also needs to set adequate, enforceable standards that will guarantee quality and safety of bottled water. Finally, in order to continue enjoying good, healthy, and tasty drinking water for years to come, we urgently need to invest into protection of ambient waters, the sources of our drinking water, and the infrastructure that delivers water to our homes. All Americans deserve to have access to good quality drinking water, with full disclosure of its sources, treatment, and potential presence of chemical contaminants. Otherwise, marketing the image of purity and not delivering on the promise leaves bottled water drinkers at risk.
Recommendations
The commercial success of bottled water in the US has been driven in part by concerns over tap water quality. And while drinking pure water is a healthy choice, bottled water is not the answer. Our study shows that bottled water is polluted with a range of contaminants, including many of the same chemical pollutants typical in municipal tap water supplies. The only effective long-term solution to ensure the safety of drinking water supplies across the country is protection and cleanup of our rivers, streams, and ground water from pollution.
Policy Recommendations
- FDA should hold the bottled water industry to the same standard of transparency that our water utilities must meet in terms of where the water comes from, how it's treated, and the residual pollution it contains. Citizens have a right to know this basic information about the bottled water that they are purchasing.
- Policy-makers should expand resources dedicated to protecting rivers, streams and ground water that serve as drinking water supplies. This is the only fail-safe way to ensure clean, safe tap water across the country.
What can consumers do?
- Drink filtered tap water Some reports show that up to 44 per cent of bottled water is just tap water – filtered in some cases and untreated in others (O'Rourke, 2008). It has also been noted that bottled water can cost up to 10,000 times more than tap water (Earth Policy Institute, 2006). A carbon filter, whether tap mounted or the pitcher variety, costs a manageable $0.31 per gallon, and removes many of the contaminants found in public tap water supplies, therefore rendering the water just as good as, if not better than, most brands of bottled water.
- Forgo the plastic bottles Plastic additives, many of which have not been fully assessed for safety, have been shown to migrate from the bottles into bottled water to be consumed (Nawrocki 2002). EWG recommends that consumers use a stainless steel bottle filled with filtered tap water to avoid these potentially harmful contaminants.
- Consumers can urge policymakers to improve and adequately fund source water protection programs The only long-term solution to our water problem is a clean water supply. This can only be achieved if policymakers enforce more stringent source water protection programs to ensure that our rivers, streams, and groundwater are adequately protected from industrial, agricultural, and urban pollution.
All Test Results
Click here to see detailed results for follow-up testing on Walmart's Sam's Choice brand and Giant's Acadia brand.
| Brand 1 (Woodstock GA)# | |
| Contaminant | Concentration |
| Total Trihalomethanes | 4.4 ppb |
| Chloroform | 3.8 ppb |
| Bromodichloromethane | 0.6 ppb |
| Dichloroacetic Acid | 2 ppb |
| Fluoride | 0.55 ppm |
| Nitrate Nitrogen as N | 0.1 ppm |
| Ammonia Nitrogen as N | 0.12 ppm |
| Isobutane* | 4.5 ppb |
| Strontium-90 | 0.5 +/- 0.4 pCi/L |
| Other water quality indicator parameters | Concentration |
| Gross Beta | 2.4 +/-0.7 pCi/L |
| Total Dissolved Solids | 32 ppm |
| Brand 2 (Washington DC)# | |
| Contaminant | Concentration |
| Nitrate Nitrogen as N | 0.23 ppm |
| Acetaldehyde | 23 ppb |
| Radium-228 | 0.6 +/- 0.7 pCi/L |
| Other water quality indicator parameters | Concentration |
| Gross Beta | 1.9 +/- 0.7 pCi/L |
| Total Dissolved Solids | 46 ppm |
| Brand 3 (Silver Spring MD)# | |
| Contaminant | Concentration |
| Acetaldehyde | 20 ppb |
| Nonanoic acid* | 0.4 ppb |
| Hexadecanamide* | 0.7 ppb |
| 3-Methyl pentane* | 0.3 ppb |
| Hexane* | 0.5 ppb |
| Methylcyclopentane* | 0.8 ppb |
| Cyclohexane* | 0.4 ppb |
| 3-Methyl heptane* | 0.4 ppb |
| Octane* | 3.3 ppb |
| Decane* | 0.7 ppb |
| Brand 4 (Silver Spring MD)# | |
| Contaminant | Concentration |
| Fluoride | 0.26 ppm |
| Total Arsenic | 1 ppb |
| Nitrate Nitrogen as N | 0.25 ppm |
| Toluene | 1.2 ppb |
| 3-Methyl pentane* | 0.8 ppm |
| Hexane* | 0.8 ppm |
| Methylcyclopentane* | 1.3 ppm |
| Other water quality indicator parameters | Concentration |
| Heterotrophic Plate Count | 480 CFU/ml |
| Gross Beta | 4.4 +/- 0.8 pCi/L |
| Total Dissolved Solids | 210 ppm |
| Brand 5 (Cloverly MD)# | |
| Contaminant | Concentration |
| Nitrate Nitrogen as N | 0.13 ppm |
| Other water quality indicator parameters | Concentration |
| Heterotrophic Plate Count | 1 CFU/ml |
| Gross Beta | 1.8 +/- 0.6 pCi/L |
| Total Dissolved Solids | 20 ppm |
| Brand 6 (Columbia MD)# | |
| Contaminant | Concentration |
| Fluoride | 0.15 ppm |
| Nitrate Nitrogen as N | 0.67 ppm |
| Acetaldehyde | 36 ppb |
| 1-Hexene* | 0.2 ppb |
| Hexane* | 0.2 ppb |
| Octane* | 0.2 ppb |
| Acetaminophen | 1.3 ppb |
| Other water quality indicator parameters | Concentration |
| Total Dissolved Solids | 46 ppm |
| Brand 7 (Oakland CA)# | |
| Contaminant | Concentration |
| Total Trihalomethanes | 23 ppb |
| Chloroform | 10 ppb |
| Bromodichloromethane | 8.5 ppb |
| Chlorodibromomethane | 4.2 ppb |
| Acetaldehyde | 11 ppb |
| 3-Methyl pentane* | 0.3 ppb |
| Hexane* | 0.7 ppb |
| Methylcyclopentane* | 0.7 ppb |
| Cyclohexane* | 0.5 ppb |
| 3-Methyl heptane* | 0.6 ppb |
| Octane* | 4 ppb |
| Decane* | 0.6 ppb |
| Total Boron | 0.09 ppm |
| Other water quality indicator parameters | Concentration |
| Heterotrophic Plate Count | 2 CFU/ml |
| Gross Beta | 4.3 +/- 0.7 pCi/L |
| Total Dissolved Solids | 18 ppm |
| Brand 8 (Oakland CA)# | |
| Contaminant | Concentration |
| Total Trihalomethanes | 5.3 ppb |
| Chloroform | 4.6 ppb |
| Bromodichloromethane | 0.7 ppb |
| Toluene | 2.2 ppb |
| Isobutane* | 3.8 ppb |
| 2-Methyl-1-propene* | 0.6 ppb |
| Naphthalene* | 0.3 ppb |
| Nonadecane* | 0.4 ppb |
| Heptadecane* | 1.2 ppb |
| Hexadecane* | 0.5 ppb |
| Acetaminophen | 1.1 ppt |
| Total Boron | 0.06 ppm |
| Other water quality indicator parameters | Concentration |
| Total Coliform | 1 MPN/100 ml |
| Heterotrophic Plate Count | 1 CFU/ml |
| Brand 9 (Oakland CA)# | |
| Contaminant | Concentration |
| Fluoride | 0.55 ppm |
| Toluene | 2.9 ppb |
| Heptadecane* | 0.3 ppb |
| 2-Methyl-1-propene* | 0.5 ppb |
| Other water quality indicator parameters | Concentration |
| Gross Beta | 5.8 +/- 0.8 pCi/L |
| Total Dissolved Solids | 22 ppm |
| Brand 10 (Stafford VA)# | |
| Contaminant | Concentration |
| Total Trihalomethanes | 22 ppb |
| Chloroform | 19 ppb |
| Bromodichloromethane | 2.7 ppb |
| Dichloroacetic Acid | 2 ppb |
| Trichloroacetic Acid | 2 ppb |
| Fluoride | 0.94 ppm |
| Nitrate Nitrogen as N | 1.7 ppm |
| o-Hydroxybiphenyl* | 1 ppb |
| (Z)-13-Docosenamide* | 1.2 ppb |
| Isobutane* | 2.3 ppb |
| 2-Methyl-1-propene* | 0.3 ppb |
| Caffeine | 51 ppt |
| 1,7-Dimethylxanthine | 10 ppt |
| Other water quality indicator parameters | Concentration |
| Gross Beta | 5.1 +/- 1.5 pCi/L |
| Total Dissolved Solids | 160 ppm |
#Locations where each brand of bottled was purchased.
*These chemicals were identified in a broad GC/MS scan that matched peaks observed in the test to a large, standard chemical library; concentrations listed are approximate estimates determined by the analytical lab.
