
 You may know that bisphenol A, a synthetic estrogen found in the epoxy coatings of food cans, has been linked to many health problems. Many companies have publicly pledged to stop using BPA in their cans. But consumers like you have had no way to know which canned foods use BPA-based epoxy. Until now.
You may know that bisphenol A, a synthetic estrogen found in the epoxy coatings of food cans, has been linked to many health problems. Many companies have publicly pledged to stop using BPA in their cans. But consumers like you have had no way to know which canned foods use BPA-based epoxy. Until now.
EWG analyzed 252 canned food brands, mostly between January and August 2014, to find out which of them packed their food into cans coated with BPA-laden epoxy. Here’s what we discovered.
78 brands used cans with BPA-based epoxy lining for all their products

31 brands used BPA-free cans for all their canned products
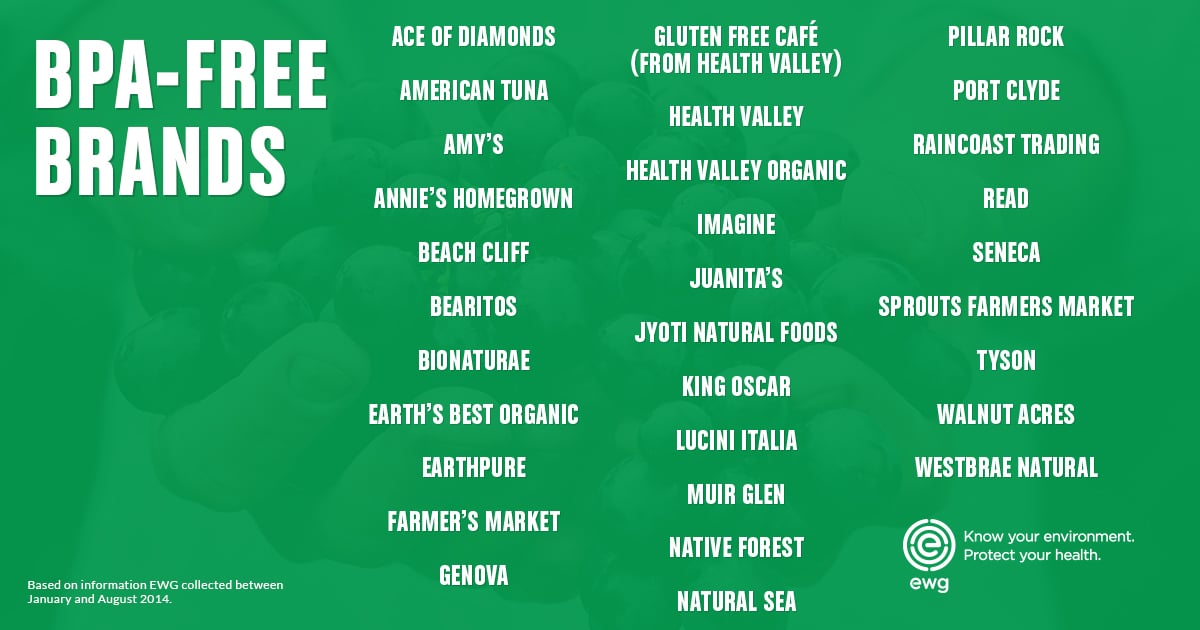
Don’t see your favorite brand on these lists? Use EWG’s Food Scores to look up individual products and see their BPA status – and so much more.
Want more information about the state of BPA in the canned food industry? Click here to read EWG’s full analysis of BPA in canned food.
Executive Summary
Many food companies have pledged publicly to stop using bisphenol A-based epoxy coatings to line their metal food cans, because BPA is a synthetic estrogen that scientists have linked to breast cancer, reproductive damage, developmental problems, heart disease and other illnesses.
But have they delivered?
The Environmental Working Group’s comprehensive survey of the American canned food marketplace has found that some food companies and brands – from small independent labels like Amy’s to global food giants like the Hain Celestial Group – offer BPA-free packaging in all product lines, but others, such as Target Corp. and Hormel Foods Corp. still use cans containing BPA. Disturbingly, consumers have no reliable way of knowing whether a canned food item is BPA-free.
EWG’s survey of 252 brands produced by 119 companies1 between January and August 2014 found that:
- 31 brands - 12 percent of the brands in our sample – used BPA-free2 cans for all canned products.
- 34 brands, or 14 percent, used BPA-free cans for one or more of their canned products.
- 78 brands, or 31 percent, used BPA-lined cans for all products. About 46 percent of the brands in this group did not say whether they were working with can suppliers or packaging manufacturers to shift to BPA-free cans or to test substitutes.
- 43 percent of all brands gave ambiguous or incomplete answers to questions about their use of BPA and/or did not respond to EWG’s queries.
- Companies that said they had eliminated BPA or were in the process of doing so did not disclose the substitutes they were using, an omission that had the effect of slowing scientific study of the possible hazards of these substitute materials. Only 13 brands volunteered even a vague description of the alternative can coatings they use.
- In the absence of a clear national standard, companies can define “BPA-free” as they wish. As a result, some products labeled BPA-free may have some amount of the chemical in can linings.
The U.S. canning industry is at a critical turning point. Strong and clear regulatory guidance and increased transparency and accessibility of information are sorely needed so that consumers can be assured that cans are truly BPA-free and that the alternatives being substituted for BPA-based epoxy are safe.
The public cannot rely on current federal laws that regulate chemicals and food additives to ensure that BPA replacement chemicals are safer than BPA-based materials.
* In June 2015, ConAgra Foods informed EWG that it has made substantial progress removing BPA from its product packaging portfolio, with plans to complete its transition to BPA alternatives by the end of July 2015.
[1] The term “company” applies to an entity with control or influence over a brand’s use, or non-use, of BPA. The brand name and the company name are not always interchangeable. The “company” could also refer to the manufacturer, the distributor, the retailer, or any combination of these.
[2] The term “using BPA-free” is meant to indicate the company is either purchasing or manufacturing cans with no BPA added to the can lining. Companies can assure that cans are BPA-free but have less control over the food ingredients. For a more detailed explanation of the term, see Appendix A.
The canned food landscape
The global canned food market was worth more than $77 billion in 2013 and is expected to reach nearly $100 billion by 2020 (Research and Markets 2013). Anchoring that growth are canned foods’ convenience, affordability and shelf-stability. Canned food can be a good source of nutrients, especially for people on tight budgets or with limited access to fresh foods. In fact, canned food is a routine part of many diets, contributing as much as 17 percent of daily nutrients for some people (FDA 2008).
Can linings, in use since the 1950s, are necessary to prevent or slow the interaction of a food with a can’s metal. Epoxy resin, made from polymers containing BPA, has been favored for can coatings because it protects against metal corrosion and holds up to the heat extremes of sterilization.
Over the last 20 years, however, a steady stream of scientific studies have documented the human health hazards of BPA, including its links to an array of illnesses and its tendency to migrate from food packaging into food.
The North American Metal Packaging Alliance, a trade group representing the metal food packaging industry, estimates that 75 percent of canned foods sold in the U.S. are lined with a BPA-based polymer (Rost in Main 2015). Residual or unreacted BPA leaches from the can into food. In 2007, EWG tested nearly 100 canned foods and baby formula and detected BPA in more than half of the samples. BPA concentrations ranged from 2 to 385 parts per billion in food (EWG 2007).
These results were confirmed and extended by testing performed by the Canadian government, the federal Food and Drug Administration, academic researchers and non-profit groups (Bemrah 2014, Cao 2008, Cao 2010, Consumer Reports 2009a, FAO/WHO 2011, Kannan and Liao 2013, National Workgroup for Safe Markets 2010, Noonan 2011, UKFSA 2001).
Nearly all Americans have measureable concentrations of BPA in their bodies; canned food is presumed to be a major source of their exposures. Harvard students who volunteered to eat canned soup daily showed a 10-fold increase in BPA concentrations in their urine compared to days that they ate fresh soup (Carwile 2011). Another study found an average 4.5 point increase in blood pressure among study participants on days they drank two servings of a beverage packaged in a BPA-based metal can as opposed to days they drank the same beverage from a glass container (BAE 2015).
Health hazards of BPA
Bisphenol A has been shown to mimic thyroid and sex hormones in people and animals. It has been associated with a wide variety of health problems, including altered brain and nervous system development and changes in the reproductive system. Much of the evidence of BPA toxicity is based on laboratory animal studies, where low exposures to BPA during pregnancy or early life can permanently affect fertility, behavior and body size and can predispose animals to later life cancers (Vandenberg 2013).
There is less than definitive evidence of BPA toxicity to people, but observational studies associate BPA exposure with a host of issues, including behavioral changes in children and diseases like obesity and heart disease.
While it is not clear how much BPA ingestion is harmful to humans, the existing evidence suggests that the developing fetus and young child are most at risk. Children cannot metabolize and excrete BPA as quickly and efficiently as adults (Edginton 2009, Ginsberg 2009). Detoxified BPA can be reactivated as it passes through the placenta to the fetus (Corbel 2015).
Regulation of BPA
In January 2010 the FDA announced that it had “some concern about the potential effects of BPA on the brain, behavior, and prostate gland in fetuses, infants and young children” (FDA 2010). It launched new efforts to reduce consumption of BPA from food by directing industry to replace BPA-based plastic in baby bottles, to shift away from BPA-based can linings for liquid baby formula and to explore alternative packaging for canned foods. FDA officials said that the agency had limited authority over BPA and other food packaging materials in use before 2000 and asked the canned food industry to submit information voluntarily about the use of BPA in food packaging (FDA 2010).
The American Chemistry Council, the chemical industry’s powerful lobbying arm, and the food canning industry have spent millions of dollars defending the use of BPA. The chemical is still permitted in most food containers, including cans. Despite ongoing campaigns by advocacy groups and lawmakers, only 13 states, the District of Columbia and a few local jurisdictions have banned BPA in reusable food containers or children’s cups and baby bottles. In 2011, EWG was instrumental in getting BPA banned from children’s foodware in California (Assembly Bill 1319, 2011). In 2012, the FDA denied a petition by the Natural Resources Defense Council to ban BPA in all food and beverage packaging (NRDC 2012).
To date, Maryland, Connecticut, Minnesota, Nevada and Vermont are the only states to have established BPA limits on the sale and distribution of one-time use food containers, including cans, of infant formula or baby food (NCSL 2015). Maryland has banned baby formula containing more than 0.5 parts per billion BPA (House Bill 4, 2011; Senate Bill 151, 2011). These state laws and FDA’s concern prompted the formula industry to stop using BPA in formula packaging by 2013 (FDA 2013).
Despite progress to lower infant exposures to BPA through food and bottles, neither the FDA nor any other U.S. regulatory agency restricts the level of BPA allowed in food and other consumer products for children and adults. Other countries, however, have established specific limits on the amount of BPA allowed in any packaged food. The European Union’s limit is 600 parts per billion of BPA per kilogram of food, and Japan’s limit is 2,500 ppb (European Food Safety Authority (EFSA) 2010, FAO/WHO 2009). Both values are much higher than the amount of BPA typically detected in canned foods (Kannan and Liao 2013) and too high to protect health or even have a meaningful effect on food production practices.
Citing BPA’s adverse effects on breast development, France has suspended production, import, export and marketing of BPA-laden packaging that comes into direct contact with food (USDA 2013). France set its safe level of BPA exposure at 0.0025 ppb per day, thousands of times lower than any other government’s. In the late 1990s Japan implemented an industry-wide shift of can coatings from epoxy resin to polyethylene terephthalate (PET) film or epoxy resin with lower BPA levels (Kawamura 2014). That led to a significant decrease in the levels of BPA in the bodies of people tested (Matsumoto 2003). It is difficult to determine a level of BPA that poses no risk to children, pregnant women and other populations based on current scientific knowledge. EWG believes that replacing BPA in can linings would lower the amount of BPA in food to less than 1 part per billion, and that development would be a significant improvement.
The FDA continues to support the use of BPA linings in food cans. Last December it released an updated safety assessment that proclaimed that “BPA is safe at the current levels occurring in foods” (FDA 2014). The agency based its determination on the results of a 90-day toxicity study. While FDA scientists concluded the study found no low-dose effects of BPA in test animals, critics point out that the animals FDA reared as an unexposed or “control” group had BPA exposures in line with its two lowest dose groups (Hunt 2014). Furthermore the agency is rushing to judgment. The 90-day study was a pilot for a multi-year, multi-million dollar toxicity investigation called Clarity-BPA, which is a partnership between the FDA, the National Institute of Environmental Health Sciences and dozens of academic researchers. The consortium presents an unprecedented scope for evaluation of BPA toxicity. FDA’s December announcement was a particularly bizarre move, considering the abundance of studies currently pending completion.
While the FDA regulates food packaging uses of BPA, the Environmental Protection Agency regulates many other uses. In 2009, the EPA named BPA one of 10 chemicals and chemical groups for which it would be creating action plans (EPA 2015a). Notably, as part of the BPA action plan, the EPA has proposed rulemaking under the Toxic Substances Control Act to identify BPA as “a substance that may present an unreasonable risk of injury to the environment on the basis of its potential for long-term adverse effects on growth, reproduction and development in aquatic species at concentrations similar to those found in the environment” (EPA 2015b). However, this proposed rule has been stalled in the Office of Management and Budget for several years (EPA 2015c).
While the proposed rule languishes in the OMB, last May California secured a landmark victory when the Developmental and Reproductive Toxicant Identification Committee of the Office of Environmental Health Hazard Assessment voted unanimously to add BPA to the state’s Proposition 65 list. California’s Safe Drinking Water and Toxic Enforcement Act of 1986, better know as Prop 65, requires a clear and reasonable warning before exposing an individual to a carcinogen or reproductive toxin on the Prop 65 list. After weighing expert testimony from public health groups including EWG, the committee classified BPA as a female reproductive toxin. It plans a separate meeting to determine which of the products that contain BPA must bear Prop 65 warning labels. The Prop 65 designation could prompt some manufacturers to switch to BPA-free can linings, even before formal labeling rules take effect.
EWG’s market survey and analysis
EWG’s survey focused on brands and companies offering classic canned foods – vegetables, fruits, juices, beans, soups, stews and other canned meals, deli goods, tomatoes, sauces, meat, fish and shellfish, canned milk, coconut milk and desserts. Previous testing of some of these products had shown that BPA could migrate from the can linings to the food at levels that posed a risk to health.
To develop its list of canned food brands and companies, EWG searched corporate and brand websites and used data and images from LabelINSIGHT® (Appendix A), a company that gathers information from American supermarkets. EWG collected information on current can lining practices from brand and company websites and social media pages and through direct phone and email communication with company representatives. The bulk of direct communication with companies took place over eight months from January to August 2014.
In all, EWG identified 252 appropriate food brands produced by 119 companies (Appendix B), including small independent companies, major global food brands and private-label brands from major supermarket chains.
Categories
EWG classified each brand’s BPA use in one of four categories:3
- Using BPA-Free Cans: A brand was classified as using BPA-free cans if it reported using no BPA in any of its canned products.
- Using BPA-Free Cans for Some Products: A brand was classified as using BPA-free cans for some products4 if it had eliminated BPA from some but not all of its canned products. (See Appendix C for a list of products in this category.)
- Using Cans With BPA: A brand was classified as using cans with BPA if none of its canned products are BPA-free.
- Unclear: A brand was classified as unclear5 if information was not available or the company-provided information was too limited to make a decisive judgment on BPA use.
Companies often have multiple brands. Brands belonging to one company sometimes have different BPA practices6.
Based on their products’ combined BPA status, EWG then placed a select group of companies surveyed into one of four groupings: Best Players, Better Players, Uncertain Players and Worst Players.
Best Players:
Companies with brands exclusively in the using BPA-free cans category7.
Based on EWG's analysis, the good news is that 31 brands and 21 companies are using BPA-free cans for at least one of their brands (Appendix D).
Only 13 companies, however, are using BPA-free cans for all their products and/or brands (Table 1).
Table 1. Best Players
| Company | Brand |
|---|---|
| American Tuna, Inc. | American Tuna |
| Amy's Kitchen, Inc. | Amy's |
| Annie's, Inc. | Annie's Homegrown |
| Euro-USA Trading Co., Inc. | Bionaturae |
| Farmer's Market Foods, Inc. | Farmer's Market |
| Juanita's Foods | Juanita's |
| Jyoti Natural Foods | Jyoti Natural Foods |
| King Oscar AS | King Oscar |
| Lucini Italia Company | Lucini Italia |
| Raincoast Trading Company | Raincoast Trading |
| Sprouts Farmers Market, Inc. | Sprouts Farmers Market |
| The Hain Celestial Group, Inc. | Bearitos, Earth's Best Organic, Gluten Free Café (From Health Valley), Health Valley, Health Valley Organic, Imagine, Walnut Acres, Westbrae Natural |
| Tyson Foods, Inc. | Tyson |
Source: Environmental Working Group, from data collected in EWG market survey and analysis, 2014
** Disclaimer. The conclusions and findings that appear on this page reflect EWG's research at the time of publication stated above. In light of evolving market conditions, subsequent product reformulations, and other factors, they may no longer be current. EWG makes no representations or warranties about any of the products that may appear on this page. EWG hereby disclaims all warranties with regard to any of the products that may appear on this page, including express, statutory, implied warranties of merchantability or fitness for a particular use.
Consumers can have confidence in the safer packaging of all products sold from these top-shelf makers.
- Amy’s Kitchen, Inc. sets the gold standard. It uses BPA-free cans for its entire line of Amy’s canned goods and makes this policy clearly known on its website (as of April 2015). It uses BPA-free labels and has provided some information about the alternatives it uses. The company has reported that its tests for BPA on its products with the new liner reveal a detection level of less than 1 part per billion, a reasonably rigorous standard that accounts for the likelihood that some food itself can test positive for BPA contamination, or the chemical can be introduced during processing.
- Another clear winner in this category is the Hain Celestial Group, an early adopter of BPA-free packaging. Hain Celestial owns Bearitos, Earth’s Best, Imagine, Walnut Acres and Westbrae Natural brands.
- King Oscar AS, which sells seafood in BPA-free tins under the King Oscar label, is another champion. According to its Facebook page, it independently tests its tins “to assure they are indeed BPA-free” (King Oscar 2014).
- Sprouts Farmers Market, Inc. shines as the only supermarket chain in our survey to offer a complete line of BPA-free canned goods, with no exceptions, under its own private label, (Sprouts Farmers Market).
- Other Best Players with excellent disclosure of their BPA-free status on their company websites include American Tuna, Inc., Euro-USA Trading Co., Inc., Farmer’s Market Foods, Inc., Lucina Italia Company and Raincoast Trading Company.
- Rounding off our Best Players list are Annie’s, Inc., Juanita’s Foods, Jyoti Natural Foods and Tyson Foods, Inc. All reported using only BPA-free cans for all canned items.
Better Players:
Companies using BPA-free cans for some of its brands and/or products.
This category spans a wide range of companies, from those that converted to using BPA-free cans for all but a single product type to those that converted just a small fraction of their canned offerings. A total of 34 brands belonging to 26 companies are using BPA-free cans for some of the line. Of these, 20 responded to our request for specific product information or had a publicly available list online. Another 15 companies reported using BPA-free cans for some types of products for every one of its brands. They provided, at a minimum, food category lists – all canned tomatoes, all canned beans, etc. Some brands went a step further, providing complete lists of full product names.
Eden Foods deserves honorable mention for its pioneering work to develop its signature BPA-free oleoresin lining. Eden also rates high for transparency. It acknowledges on its website (as of April 2015) that it uses an epoxy-based lining in its canned tomato products.
Another standout, Natural Value, Inc., has transitioned the majority of its products to BPA-free packaging. Natural Value provided EWG on April 21, 2014 with UPC codes for all of its 44 BPA-free, Natural Value canned items8 (See Appendix C). In contrast, other companies merely indicated that some of their products were in BPA-free cans but did not identify the products or categories.
- Aldi Nord’s Trader Joe’s brand packs all of its tomatoes, chicken and beef, most of its fruit, vegetables and beans and some of its soups and seafood in BPA-free cans. Trader Joe’s website (as of April 2015) clearly distinguishes which of its products are packaged in BPA-free linings and which have not yet transitioned.
- Whole Foods Market told EWG it uses BPA-free cans for all of its 365 Everyday Value canned tomatoes, fish, coconut milk and pumpkin, as well as all 365 Organic Everyday Value canned vegetables.
- Wegmans brand, from Wegmans Food Markets, Inc., earned good marks for its sizable list of BPA-free canned fruit and vegetable products and for the prominent BPA message on its website (accessed April 2015).
- Another company with superior customer communications is Crown Prince, Inc., which owns Crown Prince, Crown Prince Natural and Ocean Prince brands. The majority of its canned products use a non-BPA lacquer lining. Its message comes across consistently (as of April 2015) on its “BPA Free Cans” web page, in the individual product descriptions on its website and on the products themselves. The company provided EWG with UPC codes.
- Seneca Foods Corp., whose Read and Seneca brands use BPA-free cans exclusively, is making great progress. Its Libby’s brand uses BPA-free cans for most vegetables but reported to EWG (in August 2014) that it had not yet adopted BPA-free cans for all products. Seneca Foods deserves bonus points for providing the year that its BPA-free cans went on the market and helpful instructions on how to decipher the can coding system that identifies the year.
At the lower end of the Better Players spectrum,
- ConAgra Foods, Inc.* reported on January 22, 2014 using BPA-free cans for some products in only one of its 13 brands - Hunt's.
- Del Monte Foods, Inc. told EWG on August 13, 2014 it used BPA-free cans for some products in its College Inn, Contadina and S&W lines.
- General Mills reported on January 19, 2014 and September 4, 2014 using BPA-free cans for its Muir Glen brand but not for its Green Giant, Old El Paso and Progresso lines.
Uncertain Players:
Some 109 brands marketed by 54 companies did not make clear whether they were using BPA in their cans. Thirty-two of the 54 companies did not respond to EWG’s requests for information.
Some 22 companies responded or had website information available but failed to clearly communicate their cans’ BPA status. A number were unwilling to respond to what they deemed a too-broad inquiry. Some refused to say definitively whether some of their products had shifted to BPA-free cans unless EWG provided specific product names or lot codes identifying a particular batch of cans.
The reason typically given was that the brand or company contracts with hundreds of suppliers to produce and package its products, and the suppliers obtain their cans independently. Some companies said that some products may have multiple suppliers, and they would need specific product information to determine the supplier for any particular product. In particular:
- A spokesperson for Wal-Mart’s Great Value brand told EWG on July 31, 2014 that the company could only give information by telephone on a product-by-product basis, with a limit of three products per call, and it would need to know what store the product came from. Its website (accessed May 2015) has no information on BPA in canned food.
- Supervalu, Inc., parent company for the brands Carlita, Cowboy Billy’s, Del Pino’s, Essential Everyday, Shop ‘N Save, Wild Harvest Organic and Shopper’s Value, said on August 1, 2014 it would need to know the brand name, product type, product UPC and product lot in order to respond to EWG’s query.
A consumer trying to figure out whether a product has a liner with BPA will find that information difficult to come by and may have to buy the product first.
Many companies reported some progress in making a transition to BPA-free cans, but some were more cooperative than others in quantifying that progress and providing clear, measurable data.
For instance, in an email responding to EWG’s specific inquiry about Kraft Foods Group’s9Taco Bell brand, the company said only that it uses “an interior epoxy coating with trace amounts of BPA” in “some of (its) products packaged in metal cans.” It did not say whether this included Taco Bell products (K. McMiller, personal communication, August 7, 2014).
B&G Foods, Inc., responding to EWG’s query about its Ortega brand and six of its other brands, wrote in an email that, “Most of the cans and plastic packaging B&G Foods, Inc. uses do contain BPA” (B&G Foods consumer affairs representative, personal communication, January 23, 2014).
Campbell Soup Company responded: “We've already started using alternatives to BPA in some of our soup packaging, and we're working to phase out the use of BPA in the linings in all our canned products… We currently have millions of non-BPA cans in the market” (Campbell Soup Company representative, personal communication, August 6, 2014). Campbell Soup Company markets canned products under its Campbell’s, Pace, Swanson, V8 and SpaghettiOs brands. The Campbell’s brand has at least four lines of canned soups: Condensed Soups, Chunky, Healthy Request and Homestyle. EWG pressed for clarification as to which of its brands and products use BPA-free cans, but the company did not respond further.
Other companies that ignored EWG’s inquiries and provide no relevant information on their websites were Ahold USA, Aldi US, Albertson’s, LLC, Associated Wholesale Grocers, Rite Aid Corp., Roundy’s Supermarkets, Inc., Del Haize America, Panos brands, Teasdale Foods, IGA, Inc. and Food Lion, LLC – companies with broad reach and multiple brands. However, some companies among the Uncertain Players, including Allens, Inc.10 and The Kroger Co., are making at least some effort. Allens, Inc. said on January 23, 2014 it was commercially testing a non-epoxy lining but did not identify exact retail markets, brands, or products. Consumers who purchase the brands Allens, Butterfield, Freshlike, Sugary Sam, Trappey’s, Veg-All, Popeye Spinach, Princella or Royal Prince would have no way to know for certain whether their cans were part of the trial. Although The Kroger Co. did not provide details on the extent of their use of BPA-free cans, Kroger’s website (accessed April 2015) indicated that it was working on the issue. These two companies, and others, have given various reasons for not shifting more quickly to BPA-free cans, including:
- Suppliers and manufacturers are still researching suitable substitutes.
- Alternatives must pass regulatory approval.
- New linings must meet strict freshness, preservation and quality standards and require testing throughout a product's entire shelf life.
- Flavor concerns have arisen with some alternatives.
- There are no acceptable BPA replacements for high-acid foods such as tomatoes and some fruits and seafood.
- BPA-free cans are in short supply in some countries where products are packed.
- The limited stock of BPA-free cans leaves some companies at a purchasing disadvantage.
- The higher cost of alternatives makes it difficult to offer moderately priced products.
Worst Players:
Companies with brands in the using cans with BPA category exclusively.
In its analysis, EWG identified 78 brands (31 percent of the total survey) that still use cans lined with BPA (See Appendix B). These brands are marketed by 35 companies (29 percent of the total). Some 27 companies (23 percent) use BPA cans in all their brands or products and receive special placement on our Worst Players list (Table 2).
Table 2. Worst Players
| Company | Brand |
|---|---|
| Andre Prost, Inc. | A Taste of Thai, Coconut Milk by Andre Prost, Inc. |
| Bell-Carter Foods, Inc. | Lindsay Olives |
| Bookbinder's Foods, Inc | Bookbinders Specialties |
| Bruce's Foods Corporation | Bruce's, Casa Fiesta |
| Bush Brothers & Company | Bush's |
| Cento Fine Foods | Cento |
| Chincoteague Seafood, Inc. | Chincoteague Seafood Brand, Gordon's Chesapeake Natural |
| Culinary Collective | Matiz Gallego |
| Hormel Foods Corporation | Dinty Moore, Hormel, Hormel Chili, Peloponnese, Spam, Stagg Chili, Valley Fresh |
| Knouse Foods Co-operative, Inc. | Lucky Leaf, Musselman's |
| Look's Gourmet Food Company, Inc. | Bar Harbor |
| Mario Camacho Foods | Mario |
| McCormick & Company, Inc. | Simply Asia, Thai Kitchen |
| MegaMex Foods, LLC | Chi-Chi's, Embasa, Herdez, La Victoria |
| Musco Familiy Olive Company | Early California, Pearls |
| National Fruit Product Co. | White House Foods |
| Nestlé USA | Carnation, Libby's Pumpkin |
| Ocean Spray Cranberries, Inc. | Ocean Spray |
| Oregon Fruit Products | Oregon Specialty Fruit |
| Pea Soup Andersen's | Andersen's |
| Pinnacle Foods Group, LLC | Armour, Brooks, Duncan Hines Comstock, Duncan Hines Wilderness, Nalley |
| Rao's Specialty Foods, Inc. | Rao's Homemade |
| Red Gold, Inc. | Red Gold, Red Pack, Sacramento, Tuttorusso |
| Sokol & Company, Inc. | Solo Foods |
| Target Corporation | Market Pantry |
| The J.M. Smucker Company | Eagle Brand, Magnolia, PET |
| Topco Associates, LLC | Clear Value, Dining Out, Food Club, Full Circle, Valu Time, World Classics |
Source: Environmental Working Group, from data collected in EWG market survey and analysis, 2014
** Disclaimer. The conclusions and findings that appear on this page reflect EWG's research at the time of publication stated above. In light of evolving market conditions, subsequent product reformulations, and other factors, they may no longer be current. EWG makes no representations or warranties about any of the products that may appear on this page. EWG hereby disclaims all warranties with regard to any of the products that may appear on this page, including express, statutory, implied warranties of merchantability or fitness for a particular use.
- Hormel Foods Corp. has seven brands on EWG’s list and a 50 percent stake in another four brands.11 None are using BPA-free cans.
- All five of Pinnacle Foods Group, LLC’s brands, including Armour, Brooks, Duncan Hines Comstock, Duncan Hines Wilderness, and Nalley, use BPA-based cans.
The majority of brands and companies in the using cans with BPA category indicated that they are sensitive to consumer concerns and demand for BPA alternatives, aware of the evolving science and tracking potential changes to FDA’s or international food safety regulations, but fewer than half showed signs of making a prompt change. Some tout their removal of BPA in other products, including baby and toddler supplies, plastic shopping bags, paper receipts and frozen meal trays, but they still use the chemical for can linings.
Most provided no timeline for action. Some 18 companies representing 42 brands reported that they had begun to research or test alternatives with suppliers or were working to reduce or eliminate BPA from their packaging. But 17 companies with 36 brands made no reference to progress on this front.
- J.M. Smucker Co. said that its Eagle Brand, Magnolia and PET canned milk brands contain BPA in amounts “within the limit approved by the FDA” (J.M. Smucker Co. customer service relations, personal communication, January 15, 2014).
- Topco Associates LLC, parent company to the Clear Value, Dining Out, Valu Time, World Classics, Full Circle and Food Club brands, wrote: “The industry is actively researching alternative applications for can linings. In the meantime, we understand the concern over this particular application, and we continue to monitor the issue closely to assure that all of our packaged foods are in compliance with government regulations and industry standards” (Topco Associates, LLC consumer services team, personal communication, January 17, 2014).
- Target Corp., parent to the Market Pantry brand, responded: “Today’s standard metal food can liner contains a small amount of BPA. This particular metal can liner is commonly found within can products sold across the retail food industry. The Target owned brand food manufacturers have guaranteed Target that food products and their containers adhere to all FDA food safety requirements” (Target Corp. guest relations, personal communication, January 29, 2014).
* In June 2015, ConAgra Foods informed EWG that it has made substantial progress removing BPA from its product packaging portfolio, with plans to complete its transition to BPA alternatives by the end of July 2015.
[3] A more detailed explanation of the methods used to classify BPA-status appears in Appendix A.
[4] Brands and companies reporting some BPA-free cans were asked to provide a list of specific products to support their claims.
[5] Brands and companies that either failed to respond to EWG’s queries or responded ambiguously, coupled with a BPA status that was either unclear or unavailable through their websites or social media feeds.
[6] Thirteen companies have some differences in BPA status across their brands and were therefore not included in the tally of companies with a consistent BPA status for all of its brands. They are listed in detail in Appendix E.
[7] Companies that conducted their own can testing, and provided a limit of detection that EWG deemed unreasonably high, were not eligible for the Best Players list.
[8] Information provided by Natural Value, Inc. regarding its coconut water and pet food is not included in this figure.
[9] Communication with Kraft Foods Group, Inc. occurred prior to its merger with the Berkshire Hathaway, Inc. and 3G Capital consortium-owned, Heinz.
[10] Communication with Allens, Inc. occurred prior to its acquisition by Sager Creek Acquistion Corp. and subsequent name change to Sager Creek Vegetable Company.
[11] MegaMex Foods, LLC is a Joint Venture Between Hormel Foods Corporation and Herdez del Fuerte, S.A. de CV.
What does BPA-free mean?
EWG’s survey yielded only fragmentary information on the types of substitute can linings being adopted by food companies. Only a handful of companies or brands disclose the alternatives they are using, and the descriptions are vague. Here is a sample:
- Farmer’s Market Foods, Inc. (Farmer’s Market): Confirms information it provided to Madison, WI – based, Willy Street Co-op on September 24, 2012, that “it's a polymer liner that complies with FDA regulations.”
- Sprouts Farmers Market, Inc. (Sprouts Farmers Market): “Vinyls, polyester, and oleoresins” either in combination or alone. August 7, 2014
- Lakeside Foods – Private brand supplier for Natural Value, Inc. (Natural Value): Either “heavy single coat vinyl; single coat white modified vinyl; grey modified polyester base coat, clear modified polyester top coat; grey vinyl base coat, grey vinyl top coat; single coat modified polyester; or vinyl organosol liquid” depending on the can type.
- Annie’s, Inc. (Annie’s Homegrown): “Modified polyester material.” March 27, 2014
- Bumble Bee Foods, LLC (Beach Cliff, Bumble Bee, Wild Selections): “Blend of vinyl and polyester resins.” January 14, 2014
- Chicken of the Sea International (Ace of Diamonds, Chicken of the Sea, Genova): “Organisol polyester vinyl lining.” August 22, 2014
- The Hain Celestial Group, Inc. (pertaining to Westbrae Natural): “It is a type of food grade epoxy – it’s the simplest earth friendly coating available.”
- Edward & Sons Trading Co. (Native Forest): “It will vary from product to product, but most use a epoxy-based lacquer or a titanium dioxide-based lining.” February 5, 2014
The standout exception is Eden Foods, which is the most transparent with regard to its non-corn-based oleoresin liners: “Eden Organic Beans are packed in steel cans coated with a baked-on oleoresinous c-enamel that does not contain the endocrine disrupter chemical, bisphenol-A (BPA). Oleoresin is a natural mixture of an oil and a resin extracted from various plants, such as pine or balsam fir.” (Eden Foods 2014).
Given its expense, it’s not surprising that oleoresin hasn’t caught on more widely for low-acid foods. According to one figure, the use of oleoresin11 could increase the manufacturing cost by 21-34 percent per can (Eden Foods 2014).
As industry scrambles to find alternatives to BPA, concern has grown that without appropriate oversight, food companies will substitute structurally similar chemicals, or new chemicals with toxicity profiles equal to or worse than BPA. The FDA reviews applications for new chemicals in food packaging but has very little information about them. And since it has limited authority to regulate chemicals that were in use before 2000, BPA-free cans may be made from chemicals that have not been properly studied for long-term health effects.
In addition to BPA, other bisphenol-type chemicals have been detected in foods, including canned food. These include bisphenol A diglycidyl ether (BADGE), bisphenol F, bisphenol F diglycidyl ether (BFDGE) and bisphenol B. The most comprehensive study of American food was conducted by Chunyang Liao and Kurunthachalam Kannan of SUNY Albany in 2013 (Kannan and Liao 2013). The researchers tested 267 U.S. food samples packaged in metal cans, glass jars and cardboard and found BPA and related chemicals in many of them. Canned foods had the highest concentrations of bisphenols, with concentrations generally between 1 and 100 parts per billion. Food sold in non-metal packaging generally had less than 1 part per billion of any bisphenol. BPA was the most common chemical detected in all food types, but bisphenol F and bisphenol S were detected in many food samples, generally in lower concentrations.
An earlier study showed that BPS was as disruptive to hormone signaling as BPA and as the human estrogen estradiol. The research suggested that exposure could lead to altered cell growth and death (Viñas and Watson 2013). These findings contradicted oft-heard assertions that BPA and BPS were “weak estrogens.”
Due to their chemical structures many bisphenol chemicals have been presumed to have some hormone-disrupting potential. (Rosenmai and Pedersen 2014). A study by University of Calgary scientists found that in utero exposure to BPS (detectable in 81 percent of the U.S. population) might lead to neurodevelopmental disorders later in life. The researchers found that doses of BPS in zebrafish embryos that were a thousandth of the accepted human daily exposure led to a 240 percent increase in abnormal growth of brain cells. The authors suggested that male hormones might be particularly disturbed by this abnormal cell development (Kinch 2015).
Last year, the EPA published a report indicating that many replacement chemicals would likely have a similar risk profile to BPA (EPA 2014). That federal law does not require chemicals to be thoroughly tested (or tested at all) before they go on the market is a serious flaw in the nation’s chemical safety laws.
[11] Oleoresin may not be a suitable alternative for use with some high acid food types.
Companies do not generally label their BPA-free canned food products
Many companies informed EWG that they no longer used BPA-based metal cans, but they have not publicized this information. Some companies such as Amy’s Kitchen, Inc., Euro-USA Trading Co., Inc., Farmer’s Market Foods, Inc., Natural Value, Inc. and Crown Prince, Inc. use can labels that say “BPA-free.” Blue Marble Brands’ Field Day brand pledges on its website (accessed April 2015) to identify its BPA-free products on the label when it switches to BPA-free packaging. Faribault Foods, Inc. reports on Facebook that new labels for its S&W Beans’ organic beans were expected to come on the market May 2013.
One problem is that regulatory or industry-wide accountability measures do not exist to ensure that BPA-free labeling is credible. Companies are free to claim that their cans are BPA-free, armed with little more than certificates and assurances from their suppliers. In some cases, companies may believe they are buying BPA-free cans, but when independently tested, these cans are discovered not to be BPA-free (Hannah Stonecypher, personal communication, February 5, 2014; Vital Choice Wild Seafood & Organics, Inc. 2015; Consumer Reports 2009b), demonstrating the need for oversight.
Some companies define BPA-free loosely. Independent testing has found BPA in supposedly BPA-free cans in amounts ranging from less than 1.0 ppb to 200 ppb – the high range of BPA concentration commonly found in canned foods (Amy’s 2015, Hannah Stonecypher, personal communication, February 5, 2014).
A BPA-free label cannot mean that zero BPA will be detected in the food sample. Some BPA is found in food packaged in glass or cardboard containers (Kannan and Liao 2013) perhaps introduced during packaging or contaminating the ingredients themselves. However, a BPA-free label should mean that minimal amounts of BPA will be found in the food.
Maryland set a limit of no more than 0.5 parts per billion of BPA in baby formula, which would be ideal. BPA concentrations in food in glass or cardboard rarely exceed 1 part per billion, so this is an achievable limit.
Recommendations
For policy makers
- The FDA, EPA, European Food Safety Authority and other international regulatory bodies should determine a safe level of exposure that takes into consideration the scientific evidence of BPA’s toxicity at low doses.
- The permissible level of BPA in canned food should be no greater than 1 part per billion. These agencies should regulate labeling to ensure that it conforms to this limit.
- The FDA should re-examine all food contact substances for safety and facilitate development of new, safer alternatives as food producers work to restore public confidence in canned products. The agency must consider adverse health effects from low-dose exposures, effects on sensitive populations and aggregate exposures from various sources.
- Congress should give the FDA authority to review food packaging chemicals approved before the year 2000, as the agency requested in 2010.
- Congress should act to reform the Toxic Substances Control Act so that the EPA is better able to take timely, meaningful action to get problematic chemicals off the market.
For food companies and can suppliers
EWG recognizes and applauds efforts that many food companies are making to remove BPA from food packaging – especially in the absence of government guidance or a federal BPA standard for canned food. However the industry as a whole must move faster. All along the supply chain food producers should harness their collective bargaining power to stimulate increased production of BPA-free materials.
EWG encourages companies that have adopted BPA-free cans for some or all of their products to have the linings independently tested to verify the certificates and claims of their suppliers, using a robust limit of detection of no higher than 1 part per billion. This would provide adequate assurance that the can lining was not manufactured with BPA and that any BPA present is only a result of the chemical’s ubiquitous presence in the environment.
Consumers want clear and unambiguous information before they buy. Companies should inform their customers of their progress toward using only BPA-free cans, allowing the public to decide what companies and brands to buy.
For consumers
EWG recommends that people limit or avoid canned food. If you purchase canned foods, select one of the brands that uses a BPA-free can liner. Ask the company about the safety of the materials used as a replacement.
We urge pregnant woman and children to take special care to avoid eating food packaged in BPA epoxy cans. It can be difficult to avoid eating some canned food when dining out but worth the effort to make the switch at home.
The following steps can reduce the risk of BPA exposure:
- Substitute fresh, frozen, or dried food for canned.
- Purchase food in alternative packaging, such as glass.
- For those who cannot avoid BPA epoxy can linings, rinsing canned beans, fruit, and vegetables in water may help lower the level of BPA in the food.
- Never heat food in the can.
- Choose canned foods from EWG’s Best Players or Better Players lists of brands and companies that attest to using BPA-free linings in all or some of their canned products. Search EWG’s Food Scores for specific products.
Consumers who want to avoid BPA in canned food or want to see more of their favorite brands and varieties available in cans without BPA need to do their homework – and take action.
- Contact the company and ask for the BPA status of its product if it’s not already publicly disclosed. Urge companies that say or imply that they are shifting to BPA-free packaging to post frequent updates on their progress on their websites and through social media.
- Demand that companies disclose what’s in their can linings. Companies need to hear from consumers that they will no longer buy products with BPA epoxy can linings or substitute linings that may pose an equal or worse threat.







