August 8 2007. Laboratory tests of canned infant formula conducted by the Food and Drug Administration (FDA) and a certified commercial laboratory reveal that a plastics chemical called bisphenol A (BPA) leaches from metal can linings into formula. According to a new EWG analyses, the amount of BPA ingested by some bottle-fed infants exceeds the doses that caused serious adverse effects in animal studies.
EWG's analyses of BPA levels in ready-to-eat and concentrated formula, paired with government data on infant formula consumption show:
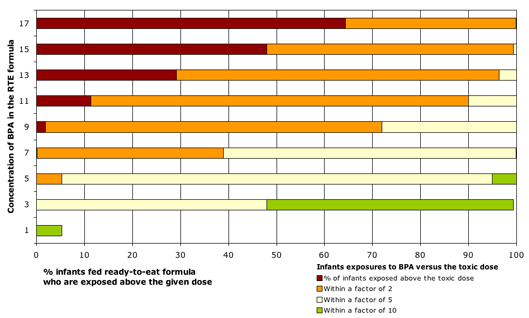
- One of every 16 infants fed ready-to-eat canned formula would be exposed to BPA at doses exceeding those that altered testosterone levels, affected neurodevelopment, and caused other permanent harm to male and female reproductive systems.
- Infants fed concentrated formula mixed with water would also be exposed to potentially unsafe amounts of BPA, in excess of standard government safety margins. While water added to concentrated formula lowers BPA concentrations in the final mixture, our analyses still show that one of every 16 infants fed concentrated formula would be exposed to BPA at doses within a factor of 2 of harmful doses.
- At the highest BPA levels found in formula, 17 parts per billion (ppb), nearly two-thirds of all infants fed ready-to-eat formula would be exposed above doses that proved harmful in animal tests (Figure 1).
These analyses, coupled with exposure estimates in other studies, demonstrate that bottle-fed infants likely face higher BPA exposures than any other segment of the population, and highlight the urgency of setting standards for this chemical to protect babies who are overexposed through canned liquid formula.
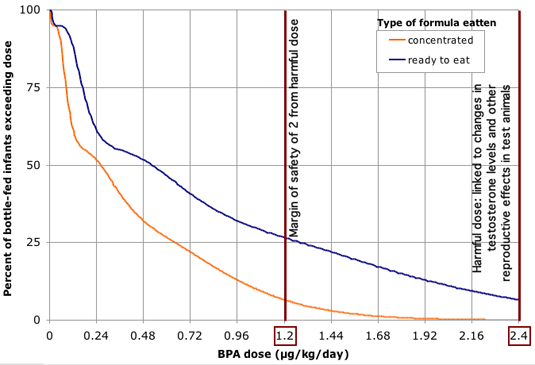
Figure 1. BPA has been found in infant formula at levels ranging up to 17 parts per billion, a concentration at which nearly two-thirds of infants would exceed doses shown to harm test animals.
Source: EWG analysis of BPA exposures based on government and commercial lab tests of BPA in formula, and formula consumption rates and body weights measured in government surveys. This graph reflects procedures described in the methodology section of this report, with the exception that exposure estimates for individual infants created in the exposure model were based on incremental, assumed BPA concentrations in formula, and grouped as a function of BPA concentration for purposes of graphical display. Estimated single-day exposures are compared against BPA dose of 2.4 ug/kg body weight / day linked in lab studies to alterations in testosterone levels and referenced as "toxic dose" in figure above (see Section 3 of this report). Note that results shown above will underpredict infants exposed above even lower doses found harmful in animal studies, including a dose of 2.0 ug/kg/day linked to permanent damage of reproductive system from in-utero exposures.
Failures to protect infants from BPA risks. FDA last assessed the safety of BPA in infant formula in 1996, based on tests of 14 infant formula samples (Bailey 1996). Dozens of peer reviewed studies published since that time reveal adverse effects of BPA at exposures dramatically lower than those known at the time to be harmful, and, significantly lower than exposures for infants drinking BPA-contaminated formula.
Yet despite scientists' dramatically altered understanding of low-dose BPA toxicity, FDA has not tested additional samples of infant formula for BPA, and has failed to reassess the safety of BPA-contaminated infant formula since its original assessment 11 years ago. FDA does not require infant formula manufacturers to test their products for BPA.
Instead, in 2006 the federal government launched a BPA health risk assessment under the National Institutes of Health's (NIH's) Center for the Evaluation of Risks to Human Reproduction (CERHR). From the outset the assessment process has been plagued by concerns over scientific credibility and conflicts of interest:
- The contractor in charge of the assessment both helped form the panel, which lacks BPA experts, and prepared the initial draft assessment for the panel's review. This contractor was subsequently fired by CERHR over concerns about potential conflicts of interest, but the panel was allowed to continue with its initial membership, working from the draft assessment prepared by the fired contractor.
- In external review comments submitted to the panel, BPA experts revealed that the CERHR assessment appears to contain nearly 300 errors of fact and interpretation; is biased, inconsistent, incomplete; and clearly fails to meet the most basic scientific standards. [see EWG's full review]
What could have been the first opportunity in a decade to advance public health protections for this problematic chemical instead ended in CERHR issuing a final assessment on August 8, 2007 that fails to support stronger public health safeguards for bottle-fed infants, pregnant women, and other at-risk populations.
BPA has been detected in thousands of people worldwide, including 93 percent of 2,500 people in the United States. More than 100 peer-reviewed studies have found BPA to be toxic at low doses, some similar to those found in people, yet not a single public health agency has updated safety standards to reflect this low-dose toxicity.
This country's toxics law, the Toxic Substances Control Act, fails to require that chemical companies prove thier products are safe before they are sold, even when these chemicals end up in people's bodies, as is the case for BPA. This law was passed in 1976, and 31 years later is the only major public health and environmental statute in this country that has never been updated. This panel certainly has not done their part to help fill the gaps in this broken system of public health protections.
As a result of these policy gaps, BPA is now one of the most widely used industrial chemicals, is found at unsafe levels in people, is allowed in unlimited quantities in a broad range of consumer products including infant formula, and is entirely without safety standards. BPA provides irrefutable proof that our system of public health protections must be strengthened to protect children and others most vulnerable to chemical harm.
Resources
Lab tests of canned food — BPA contamination in more than half of 97 name-brand canned foods: https://www.ewg.org/research/timeline-bpa-invention-phase-out
EWG comments to CERHR summarizing nearly 300 errors of fact and interpretation in BPA assessment identified by BPA experts: https://www.ewg.org/news/testimony-official-correspondence/ewg-letter-cerhr-re-interim-draft-report-bisphenol
BPA in infants and others
BPA is a widespread pollutant in the food supply and in people. Recent research reveals that BPA is a widespread human pollutant, contaminating not only infants through their exposures to BPA-contaminated formula, but also more than 90 percent of thousands of people tested worldwide, from exposures to contaminated canned foods among many other potential sources. As with infant formula, in canned foods such as soups, pasta, and vegetables, BPA leaches from the can liner into the food, and poses particular risks to women of childbearing age and young children.
BPA in food. Environmental Working Group (EWG) conducted independent laboratory tests of 97 cans of name-brand fruit, vegetables, soda, and other commonly eaten canned foods to detect the presence of BPA. EWG tests found that of all foods tested, chicken soup, infant formula, and ravioli had BPA levels of highest concern. Just one to three servings of foods with these concentrations could expose a pregnant woman or child to levels of BPA that caused serious adverse effects in animal tests. In addition, two of six cans of infant formula tested contained quantifiable amounts of BPA; the exposure that an infant might receive from canned formula, given his or her small size and limited food sources, makes the level of contamination in these cans particularly troublesome.
BPA in people. In 2005, researchers from the CDC published results from a study of urine samples from 394 adults; these scientists found BPA in 95% of samples (Calafat et al. 2005). In follow-up testing, CDC tested over 2,500 people for BPA and found detectable levels in 93% of those tested. In general, BPA exposures estimated using this new, larger sample of Americans are higher than previous estimates. Median exposures for all 1,664 adults in this study are 35% higher than that reported from the 394 participants in the earlier survey.
This large study of over 2,500 people reveals that young people are generally exposed to higher levels of BPA than adults; median BPA exposure for 260 children ages 6 to 10 was 5 times higher than for adults (0.176 ug/kg body weight/day vs. 0.035 ug/kg body weight/day). In addition, women had higher exposures than men; women of child- bearing age (18-40) had levels that were 43% higher than those found in men. CDC did not include infants in their study group, a critical gap given that concentrations in infant formula indicate that bottle-fed infants may be one of the most highly exposed populations.
BPA has also been found in umbilical cord blood in several studies, indicating that the placenta doesn’t offer protection from exposure to this chemical (Kuroda et al. 2003; Schonfelder et al. 2002; Ikezuki et al. 2002). This is especially concerning, since many BPA studies find that the developing fetus is especially vulnerable to low doses of this chemical. In addition, BPA has also been found in breast milk, also a concern given that BPA has been shown to affect the reproductive system of lab animals in infancy (Kuroto-Niwa et al. 2007; Ye et al. 2006).
Risks at low doses
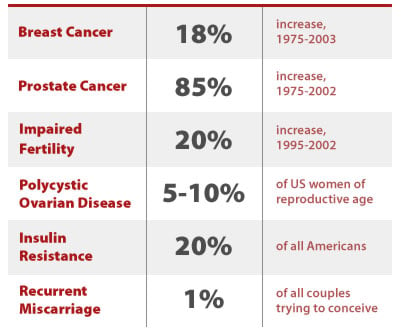
In animal studies, BPA has been linked to a variety of prevalent diseases, many of which are increasing in the United States and are taking a major toll on our collective health. These include breast and prostate cancer, obesity, and infertility. The United States is noted to have one of the highest incidence rates for breast and prostate cancers in the world; lifetime risk for these cancers has steadily risen over the last two decades and currently stands at 1 in 8 for breast cancer in women and 1 in 6 for prostate cancer in men.
In addition, obesity is also on the rise in the US and is regarded to be an epidemic by public health experts. More couples are also reporting difficulties with infertility, with a recent CDC study finding a 20% increase in the last decade in the number of couples who report having difficulty conceiving.
What is most worrisome about these animal studies is that the doses of BPA that are being used are extremely low and in the range of the levels that have been found in people. In other words, the levels of BPA that are being found in people have been linked in animal studies with serious medical conditions that affect the health and wellbeing of millions.
BPA's toxic effects in lab animals are on the rise and common in people
Many studies confirm BPA's low-dose toxicity across a diverse range of toxic effects
| Daily BPA exposure (ug/kg body weight-day) | Toxic effect | Study details | Reference |
|---|---|---|---|
| 0.0001 | alterations in cell signalling pathways on the cell surface that control calcium eflux in cells | in-vitro study which compared activity of BPA and other hormone disruptors | Wozniak 2005 |
| 0.025 | persistent changes to breast tissue, predisposes cells to hormones and carcinogens | fetal exposure, osmotic pumps, changes noted a 6 months of age | Munoz-de-Toro 2005 |
| 0.025 | permanent changes to genital tract | fetal exposure, osmotic pumps | Markey 2005 |
| 0.2 | decrease antioxidant enzymes | adult exposure, oral | Chitra 2003 |
| 0.25 | altered growth, cell size and lumen formation in mammary epithelium of mouse fetuses. | exposure during pregnancy w/osmotic pumps | Vandenberg 2007 |
| 2 | increased prostate weight 30% | fetal exposure, oral route | Nagel 1997 |
| 2 | increased aggression at 8 weeks of life | fetal exposure, oral route | Kawai 2003 |
| 2.4 | Decreased time from vaginal opening to first estrus, possibly earlier puberty | fetal exposure, oral route | Howdeshell 1999 |
| 2.4 | lower bodyweight, increase of anogenital distance in both genders, signs of early puberty and longer estrus. | fetal exposure, oral route | Honma 2002 |
| 2.4 | decline in testicular testosterone | fetal and neonatal exposure, gavage | Akingbemi 2004 |
| 2.5 | breast cells predisposed to cancer | fetal exposure, osmotic pumps | Murray 2006 |
| 2.5 | immune system impacts | oral exposure | Sawai 2003 |
| 10 | prostate cells more sensitive to hormones and cancer | infant oral exposure, 3 day duration | Ho 2006 |
| 10 | prostate cells more sensitive to hormones and cancer | fetal exposure, oral route, short duration | Timms 2005 |
| 10 | insulin resistance develops in 2 days, chronic hyperinsulinemia at day 4 | subcutaneous injection, short duration exposure | Alonso-Magdalena 2006 |
| 10 | decreased maternal behaviors | fetal and neonatal exposure, oral route | Palanza 2002 |
| 20 | damage to eggs and chromosomes | fetal exposure, osmotic pumps | Hunt 2003 |
| 20 | damage to eggs | fetal exposure, osmotic pumps | Susiajro 2007 |
| 20 | brain effects - disrupted neocortical development by accelerating neuronal differentiation and migration | single injection | Nakamura 2006 |
| 30 | reversed the normal sex differences in brain structure and behavior | oral during gestation and lactation | Kubo 2001 |
| 30 | hyperactivity | oral | Ishido 2004 |
| 50 | EPA RfD | EPA's 'safe exposure level, based on outdated, high dose studies and a 1000-fold margin of safety | EPA 1998 |
BPA concentrations are expressed in parts per billion (ppb) by weight (micrograms of BPA per kilogram of food).