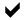Consumers instantly recognize them as household miracles of modern chemistry, a family of substances that keeps food from sticking to pots and pans, repels stains on furniture and rugs, and makes the rain roll off raincoats. Industry makes use of the slippery, heat-stable properties of these same chemicals to manufacture everything from airplanes and computers to cosmetics and household cleaners.
But in the past five years, the multi-billion dollar “perfluorochemical” (PFC) industry, which underpins such world-famous brands as Teflon, Stainmaster, Scotchgard and Gore-Tex, has emerged as a regulatory priority for scientists and officials at the U.S. Environmental Protection Agency (EPA). The PFC family is characterized by chains of carbon atoms of varying lengths, to which fluorine atoms are strongly bonded, yielding essentially indestructible chemicals that until recently were thought to be biologically inert. No one thinks so now.
A flood of disturbing scientific findings since the late 1990s has abruptly elevated PFCs to the rogues gallery of highly toxic, extraordinarily persistent chemicals that pervasively contaminate human blood and wildlife the world over. As more studies pour in, PFCs seem destined to supplant DDT, PCBs, dioxin and other chemicals as the most notorious, global chemical contaminants ever produced. Government scientists are especially concerned because unlike any other toxic chemicals, the most pervasive and toxic members of the PFC family never degrade in the environment.
The U.S. EPA peremptorily forced one member of this family off the market in 2000: PFOS, the active ingredient used for decades in the original formulation of 3M’s popular Scotchgard stain and water repellent. Shortly thereafter, 3M also stopped manufacture of a related perfluorochemical, called PFOA, that is now under intense regulatory pressure at EPA. 3M formerly sold PFOA to DuPont, which has used PFOA for half a century in the manufacture of Teflon. (DuPont now now makes the chemical itself at a new facility in North Carolina.) Alarmed by findings from toxicity studies and by the presence of PFOA in the blood of more than 90 percent of the U.S. population, EPA is expected to announce initial steps to regulate the chemical in early April (2003).
This report provides the first, comprehensive review ever published of the pollution and health risks posed by PFCs, with special reference to PFOA. It is based on a review of 50,000 pages of regulatory studies and government documents obtained from EPA; internal documents from DuPont and 3M disclosed in ongoing litigation; and an examination of a growing body of independent studies on the toxicity and environmental occurrence of PFCs.
This report also explains how major companies like 3M and DuPont, who endlessly boast about their scientific prowess, could get away with permanently contaminating the entire planet for decades amid assurance from the chemical industry that it practices “responsible care” with respect to public health and the environment.
PFOA and other PFCs come from common products in every home
April 2003
Non-stick pans, furniture, cosmetics, household cleaners, clothing, and packaged food containers can all contain PFCs, many of which break down into PFOA in the environment or in the human body. The brand names are well-known: Teflon, Stainmaster, Scotchgard, SilverStone, and others. PFCs are also used in a vast array of industrial products and processes.
Teflon and PFOA: Teflon itself is not PFOA (C8), but PFOA is used to manufacture Teflon and is released to the air, along with other PFCs, when Teflon cookware is heated to broiling temperatures [Extract]. PFOA is also emitted to air and water at Teflon manufacturing plants.
Other common consumer products and PFOA (fluoropolymers and telomer alcohols): PFOA also comes from products designed to repel soil, grease, and water, including carpet and furniture treatments, food wraps, sprays for leather, shoes and other clothing, paints and cleaning products - and from products like shampoo and floor wax, where PFCs are used as surfactants. These PFC products include formulations like Stainmaster fabric protection and Zonyl paper protection, and are made with chemicals that break down into PFOA and related chemicals in the environment and inside the body. [Extract | Full Document]
Industrial pollution and PFOA: Chemical companies like DuPont and 3M have not been required by law to monitor or report emissions of PFOA, PFOS or other PFCs into air, water or landfills because the chemicals are completely unregulated—so all emissions are legal. Industry studies submitted to EPA provide the companies’ partial estimates of pollution loadings, but only for recent years and only from manufacturing plants, not from “downstream” industrial users. From these documents we know that tons of PFOA have been released annually as air and water pollution from DuPont and 3M plants in West Virginia, North Carolina, Minnesota and Alabama; and from carpet, clothing, and paper industries in Georgia, North Carolina, and other places. In 1999 alone, DuPont released over 40 tons (86,806 pounds) of PFOA into the air and the Ohio River from its Washington Works Teflon production facility in West Virginia. The company now boasts of having reduced those loadings to 10 tons (20,168 pounds) in 2002.
3M’s Original Scotchgard and PFOA: Scotchgard products made by 3M before the year 2001 break down into PFOA, among other chemicals, in the environment [Extract | Full Document]. The Environmental Protection Agency forced 3M to alter its Scotchgard formulation because chemicals in the product were found to be toxic and persistent in the environment and the human body. The public record contains little information on the new Scotchgard formula. 3M is using PFBS, a sister chemical to PFOS, as a replacement to PFOS based chemistry for some products [Full document]. PFBS does not break down. If 3M's replacement chemistry is based on telomer alcohols, it likely breaks down into PFOA as well.
Other PFCs: PFOA is only one of many terminal breakdown compounds of household products that contain PFCs, and only one of 15 PFCs known to pollute human blood. Some other persistent compounds that come from consumer products and are found in human blood include PFOA's chemical "sisters" that are characterized by carbon chain lengths either longer or shorter than PFOA's 8-carbon chain. Another group of PFCs found in consumer products breaks down into PFOS-like chemicals, the perfluorinated sulfonates that formed the basis of 3M's original Scotchgard formulation.
PFOA is a pervasive pollutant in human blood, as are other PFCs
April 2003
DuPont, 3M and other PFC manufacturers had ample indications decades ago that PFOA and other perfluorochemicals contaminate the blood of the general U.S. population. How and why they ignored the warning signs is one of the more distrubing chapters in the unfolding tragedy of PFC pollution.
In studies the 3M Company submitted to the government in 2001, scientists reported finding PFOA in the blood of 96 percent of 598 children tested in 23 states and the District of Columbia. [Extract | Full Document] Although this remains the largest study of children's blood, it was not the first. In 1981 DuPont found PFOA in umbilical cord blood from one baby and blood from a second baby born to female workers at its Teflon plant in Parkersburg, West Virginia. Among seven pregnant workers monitored by DuPont, two gave birth to babies with birth defects - one an "unconfirmed" eye and tear duct defect, and one a nostril and eye defect [Full Document]. That same year, DuPont reassigned 50 women from the plant [1].
Between 1972 and 1989, no less than nine studies were published on levels of PFCs in blood from the general population. PFOA was first tentatively identified in human blood as early as 1976, about the time PCBs were banned. In studies conducted in the past six years, industry scientists have detected PFOA in the vast majority of samples tested from nearly 3000 people in the US, including blood samples from 598 children, 238 elderly Washington State residents, and approximately 2000 blood bank donors.
Scientists have now found 15 PFCs in human blood - every PFC for which they have tested. In industry's 2001 study of six PFCs in human blood, scientists found four at higher levels in children than in adults. And children showing the highest levels were within the range of what has been measured in 3M workers [3][Extract | Full Document]. Recent laboratory studies heighten concerns about the effects these chemicals might have on children's health and development [4]. PFOA was found at similar levels in children and adults, but children may be at higher risk to the incremental effects of PFOA exposures simply because their bodies carry higher levels of other PFCs with toxicities similar to that of PFOA.
Once introduced, PFOA circulates in the body for years. If new exposures to PFOA could somehow be stopped, the body would require an estimated 4.4 years to excrete half the mass of PFOA accumulated in organs and tissues [2]. But since humans appear to be exposed frequently, perhaps daily, through consumer products and environmental contamination, the fact that the body can slowly excrete PFOA has less relevance to human health than the fact that PFOA appears to be continually reintroduced.

Even if PFOA were banned from use, its concentration could continue to build in the environment and in human blood. Over time other fluorinated compounds in common consumer products will degrade to their terminal breakdown product - PFOA. The rate at which this transformation will occur is not known, but could require decades or centuries, given what is known about degradation rates of other precursor PFCs. Humans could be exposed to ever-increasing amounts of PFOA through exposure routes driven by environmental contamination (tap water, food, and air, for example), even as post-ban exposures to PFOA from consumer products decline.
Fifteen PFCs have been found in human blood. In nearly 20 studies conducted between 1972 and 2002, scientists have found all 15 PFCs targeted in lab analyses, including:
- Eight chemicals in the PFOS family (the sulfonates), most stemming from the original Scotchgard formulation. Scientists have detected PFOS itself, and five chemicals that appear as intermediates in the breakdown chain from the active Scotchgard ingredients to the terminal breakdown product (PFOS), including M570, M566, PFOSAA, and PFOSA. They have detected THPFOS, a chemical structurally related to PFOS, and PFHS and THPFDS, cousins of PFOS with a 6- and 10-carbon chains, respectively, instead of an 8-carbon chain like PFOS. [24]. [Excerpt | Full Document]
- Seven chemicals in the PFOA family (the carboxylic acids), most stemming from Teflon products, and telomer alcohol and acrylate polymer products like Stainmaster and Zonyl chemicals used in food packaging. Scientists have found PFOS itself, and chemical sisters to PFOA characterized by carbon chain lengths ranging from 6 to 12 (chemicals referred to as C6, C7, C9, C10, C11, and C12). [Excerpt | Full Document]
Evidence of blood contamination in the 1970s. Early work to define human contamination by 3M chemicals was done not by the company but by dentists researching fluoride in the human body. Dr. Donald Taves of the University of Rochester's School of Medicine and Dentistry reported a surprising finding in a workup of a sample of his own blood. His analyses showed that some of the fluoride in his blood appeared to be organic, and unrelated to the types of fluoride added (controversially) to public drinking water supplies for purposes of dental hygiene.
In 1976 Dr. Taves and his collaborators tentatively identified one of the organic fluorines in human blood samples as one of 3M's perfluorinated organics - PFOA, or perfluorooctanoic acid [5]. The authors speculated that multiple perfluorocarbons appeared be present in human blood, and that some might be branched, or sulfonated (another group of 3M's perfluorochemicals). The authors presciently speculated on the source of these chemicals in blood:
"These findings suggest that there is widespread contamination of human tissues with trace amounts of organic fluorocompounds derived from commercial products.... A series of compounds having a structure consistent with that found here for the predominant form of organic fluorine in human plasma is widely used commercially for their potent surfactant properties. For example, they are used as water and oil repellants in the treatment of fabrics and leather. Other uses include the production of waxed paper and the formulation of floor waxes...The prevalence of organic fluorine in human plasma is probably quite high since 104 of the 106 plasma samples tested here and all 35 in an earlier study... had measureable quantities.... Computer assisted literature searches using Medline, Toxline and Chemcon developed no information on [metabolism and toxicology of these chemicals]. This was surprising with respect to the widespread commercial use of such compounds." [5].
A total of nine studies were completed between 1972 and 1989 quantifying levels of organic fluorine in human blood - in the general population in the US, Argentina, China, and Japan [6 (p.13)]. In 1976 3M began testing for - and finding - PFOA in workers' blood [6 (p.13)], but for 31 years after Taves' initial discovery, industry apparently and perhaps willfully failed to pursue studies that almost surely would have defined and confirmed the extent to which PFOA had contaminated the general population.
Despite the many findings of PFCs in human blood available in the peer-reviewed literature, 3M's medical director expressed "surprise" [7] when 1997 company-sponsored tests showed PFOS not only in workers' blood, but also in supposedly clean blood - samples from U.S. blood banks that were to be used as control samples in the tests. 3M withheld the findings for nine months while conducting confirmatory tests.
3M began a series of studies at their environmental laboratory to define the extent to which their chemicals contaminate people and the environment. In March and April 1998 3M's environmental lab finalized ten new studies that confirmed the presence of PFCs in the blood of the general population.
Perfluorochemicals were found in 18 pooled blood bank samples from all across the U.S. and in blood samples taken in the remote Linxian and Shandong provinces in rural China in samples drawn in 1984 and 1994. In only one group of samples were PFCs not found: ten archived blood samples taken from Korean War-era U.S. military recruits sampled between 1948 and 1951, about 10 years before PFOS chemicals went into commercial production.
|
Perfluorochemicals are found in blood samples from these groups |
PFC detected |
Not detected in |
|
|
1948-1951 - Korean War era U.S. military recruits, 10 pooled samples |
|
|
1957 - Sweden, 10 individual samples |
PFOS |
|
|
1969-1971 - Michigan, 5 individual samples from a breast cancer study |
PFOS |
|
|
1971 - Sweden, 10 individual samples |
PFOS |
|
|
1976 - U.S., 6 pooled samples from heart disease study |
PFOA |
|
|
1980 - U.S., 3 pooled samples from heart disease study |
PFOS |
|
|
1984 - Linxian, rural China province, 6 individual samples* |
PFOS |
|
|
1985 - U.S., 3 individual samples from heart disease study |
PFOS |
|
|
1994 - Shandong, rural China province, 6 individual samples* |
PFOS |
|
|
1995 - U.S. children in 23 states plus District of Columbia |
PFOS, PFOA, PFHS, PFOSA, PFOSAA, M570, M556 |
|
|
1999 - U.S. elderly in Seattle, Wash. |
PFOS, PFOA, PFHS, PFOSA, PFOSAA, M570, M556 |
|
|
2000 to 2002 - U.S. blood from commercial and blood bank sources |
PFOS, PFOA, PFOSA, PFOSAA, M570, M556, C6, C7, C9, C10, C11, C12, THPFOS, THPFDS |
*Note: In 3M documents AR226-0178[8] and AR226-0202[9], the levels of PFOS found in the 1984 and 1994 rural China samples are not distinguished separately. Three of the 12 individual samples from the 2 sample groups were composited for a total of 9 samples analyzed. PFOS was found in 3 of these 9 samples at levels between 1 and 5 ppb.
Source: AR226-0178[8], AR226-0202[9], and AR226-0036[10].
PFCs in children's blood. On May 15, 2001, three years to the day from 3M's first "notice of substantial risk,"[11] alerting EPA to the widespread contamination of humans and wildlife with their Scotchgard chemical, PFOS, 3M's Medical Director Larry Zobel gave EPA more significant news - some perfluorochemicals appear to be far more prevalent in children than in adults, and in some children, levels are as high as in 3M plant workers [3].
3M had based all prior blood tests for the general population on pooled samples from blood supply warehouses. In a new series of tests, 3M analyzed individual samples from 598 children ages 2 through 12 from 23 states and the District of Columbia, and from 600 adults (primarily ages 20-69) from six blood banks across the U.S. 3M's May 15th 2001 report to EPA showed that of the six PFCs found in the blood samples, four are found at higher levels in children than in adults [EWG Extract | EWG Full Document]. And children showing the highest levels are within the range of what has been measured in 3M workers [3]. Recent laboratory studies [4, 12] heighten concerns about the effects these chemicals might have on children's health and development. 3M's May 15, 2001 report to EPA shows that:
- All 598 children and 883 adults tested have organic fluorinated chemicals in their blood. For many individual perfluorochemicals, some people have levels below the level of quantification. However, every person sampled thus far has detectable levels of organic fluorine. This type of fluorine is unrelated to the fluoride that is added to public drinking water supplies and is considered to be a surrogate measure for the presence of perfluorochemicals [13, 14].
- Children showing the highest levels are within the range measured in 3M plant workers. PFOS was found in the group of children tested at a maximum concentration of 515 ppb. In 3M's Sumitomo plant in Japan, the highest level measured in plant workers in a 1999 study was 628 ppb [15]. Notably, the U.S. Environmental Protection Agency found that workers' exposures to PFOS are dangerously close to the levels at which adverse effects are reported in studies of laboratory animals, in what EPA refers to as a potentially unacceptable "margin of exposure" [16]. 3M's new blood study for the first time puts some children in a range overlapping with worker exposures. The same is true for PFOA. At the 3M plant in Decatur, AL, worker blood levels of PFOA ranged from 40 to 12,700 ppb in 2000, while PFOA was detected in children as high as 56 ppb [17][Extract | Full Document].
- Some children have levels of perfluorochemicals in their blood at far higher levels than initial tests had shown. A 1999 pilot study of 12 children showed a maximum concentration of PFOS in sera of 115 ppb; the new study puts the maximum at 515 ppb. In the 1999 study, PFHS was found at a maximum of 100 ppb. In 3M's 2001 study, PFHS was found at more than seven times this level (a maximum of 712 ppb). Children at the highest levels of exposure show concentrations up to 48 times those of an average child.
- Levels are higher in children than in adults in the general population. Of the six PFCs found in the blood samples in 3Ms 2002 study, four are found at higher levels in children than in adults (PFHS, PFOSAA, M570, and M556)[EWG Extract | EWG Full Document]. Levels of PFHS are notably higher in children than in adults. In short-term laboratory animal studies, PFHS causes cellular damage to the thyroid and disrupts the ability of cells to communicate with each other, just like PFOS and PFOA or chemicals that break down into PFOA.
- One of the perfluorochemicals, M556, is far more prevalent in children than adults. 3M found this chemical in every one of the 598 children tested, but in fewer than five percent of the adults tested. M556 is an intermediate breakdown product of Scotchgard chemicals used in fabric and carpet protection, as the body converts the chemicals into PFOS. This result points to the possibility that children are exposed to higher levels of Scotchgard compared to adults, perhaps because of different behavior patterns. Alternatively, children could metabolize these chemicals more slowly than adults, which would lead to children being exposed to perfluorinated metabolites for longer periods of time [22].
PFCs may be even longer-lived in the human body
than previously suspected.
The 2001 children's blood study came when 3M was also backtracking on claims of how long-lived these chemicals are in the human body. 3M's estimates of the half-life of PFOS in the human body (the time it takes humans to excrete half of the amount of the chemical in their bodies) have oscillated between about one and nine years. In a 2002 study based on serum levels of PFOS in nine former 3M plant employees, 3M scientists estimate that the chemical's half-life in people is 8.7 years [2] [Extract | Full Document].
In the same group of former employees, 3M has most recently estimated a human half-life of 4.4 years for PFOA [2]. The estimated human half-life has increased as 3M has tracked workers more thoroughly [2, 23] [Extract | Full Document].
One peculiarity of perfluorochemicals is that the half-life in the body is less relevant to health concerns than it is for other chemicals. Scientists have found no mechanism by which PFOA and other terminal PFCs can be broken down in the environment. This means that every PFOA molecule on the planet is here to stay. Opportunities for humans (and other animals) to be exposed continuously to PFOA would continue even if it were banned.
References
[1] Environmental Working Group (EWG). 2002. DuPont Hid Teflon Pollution For Decades. Available online at https://www.ewg.org/policymemo/20021113/20021213.php.
[2] Burris, JM., Lundberg, JK., Olsen, G., Simpson, C and Mandel, J (2002). Determination of serum half-lives of several fluorochemicals: Interim Report #2. Study Sponsor: 3M Company, Corporate Occupational Medicine Department, US EPA AR226-1086.
[3] 3M. 2001. TSCA 8(e) supplemental notice for sulfonate-based and carboxycylic-based fluorochemicals -Docket numbers 8EHQ-1180-373; 8EHQ-1180-374; 8EHQ-0381-0394; 8EHQ-05980373. U.S. EPA Administrative Record AR226-1019.
[4] York, RG (2002). Oral (gavage) two-generation (one litter per generation) reproduction study of ammonium perfluorooctanoate (APFO) in rats. Report prepared for 3M, St. Paul, MN by Argus Research (Horsham, PA). Sponsor's Study No. T-6889.6., Reviewed in US EPA AR226-1092.
[5] Taves, DR., Guy, WS and Brey, WS. 1976. Organic fluorocompounds in human plasma: prevalance and characterization. In Biochemistry involving carbon-fluorine bonds. A symposium sponsored by the Division of Fluorine and Biological Chemistry at the 170th meeting of the American Chemical Society. Chicago, IL. August 26, 1975ed. Washington DC, American Chemical Society. Vol.: 117 - 134.
[6] 3M. 1999. Perfluorooctane Sulfonate: current summary of human sera, health and toxicological data. U.S. EPA Administrative Record AR226-0548.
[7] 3M. 2000. Charles Reich, Executive Vice President of Specialty Material Markets for 3M: "It was a complete surprise that it [PFOS] was in the blood bank supplies." The Washington Post, May 17, 2000.
[8] 3M. 2000. Laboratory composite report: analytical reports of data for fluorochemical analysis in human sera. U.S. EPA Administrative Record AR226-0178.
[9] 3M. 2000. Composite analytical laboratory report on the quantitative analysis of fluorochemicals in environmental samples: Report Number FACT GEN-021, GEN-024, GEN-030, GEN-033, LRN-W2491, W2845, W3197, EOO-1386. U.S. EPA Administrative Record AR226-0202.
[10] 3M. 1998. Working memorandum on data quality assessment [of the mean and range of PFOS (ppb) levels in current and historical human populations]. U.S. EPA Administrative Record AR226-0036.
[11] 3M. 1998. TSCA 8(e) supplemental notice: sulfonate-based and carboxycylic-based fluorochemicals -Docket numbers 8EHQ-1180-373; 8EHQ-1180-374; 8EHQ-0381-0394; 8EHQ-05980373. U.S. EPA Administrative Record AR226-0540.
[12] Christian, MS., Hoberman, AM and York, RG. 1999. Oral (gavage) fertility, developmental and perinatal/postnatal reproduction toxicity study of PFOS in rats. Conducted for 3M (St. Paul, MN) by Argus Research Laboratories, Inc. Protocal Number 418-008, Study Number T-6295.9 Reviewed in OECD 11-21-02 Hazard Assessment of Perfluorooctane Sulfonate (PFOS) and its Salts.
[13] Ubel, FA., Sorenson, SD and Roach, DE. 1980. Health status of plant workers exposed to fluorochemicals--a preliminary report. Am Ind Hyg Assoc J 41(8): 584-9.
[14] Gilliland, FD and Mandel, JS. 1996. Serum perfluorooctanoic acid and hepatic enzymes, lipoproteins, and cholesterol: a study of occupationally exposed men. Am J Ind Med 29(5): 560-8. Reviewed in US Environmental Protection Agency Administrative Record AR226-1137 (pages 153-155; PDF page 50-52).
[15] Burris, J., Olsen, GW., Mandel, JH and Schumpert, JC. 1999. Determination of serum flurochemical levels in Sumitomo 3M employees. Final Report. 3M Company, 3M Medical Department, Epidemiology, 220-3W-05, FYI-0500-01378. Reviewed in OECD Hazard Assessment for PFOS (Available online here).
[16] US EPA (US Environmental Protection Agency). 2000. Email message from Charles Auer (EPA) to OECD. U.S. EPA Administrative Record AR226-0629.
[17] Environmental Protection Agency (EPA). 2002. Revised draft hazard assessment of perfluorooctanoic acid and its salts, November 4, 2002. U.S. EPA Administrative Record AR226-1136.
[18] 3M. 2002. TSCA 8(e) substantial risk notice on: perfluorohexane sulfonate potassium salt (no CAS number). U.S. EPA Administrative Record AR226-1138.
[19] Hu, W., Jones, PD., Upham, BL., Trosko, JE., Lau, C and Giesy, JP. 2002. Inhibition of gap junctional intercellular communication by perfluorinated compounds in rat liver and dolphin kidney epithelial cell lines in vitro and Sprague-Dawley rats in vivo. Toxicol Sci 68(2): 429-36.
[20] DuPont. 2002. The updated copy of DuPont Product Stewardship on December 17, 2001. U.S. EPA Administrative Record AR226-1069.
[21] DuPont Haskell Laboratory. 2002. Results of an oral gavage combined 90-day repeated dose and one-generation reproductive toxicity study in rats for poly (oxy-1,2-ethanediyl) alpha-hydro-omega-hydroxy- ether, with alpha-fluoro- omega (2-hydroxyethyl) poly (difluoromethane) (1:1) (telomer B monoether)(CAS Number 65545-80-4; non-HPV). US Environmental Protection Agency: Toxic Substance Control Act (TSCA) Section 8(e) Submission Received from 10/15/01 thru 12/07/01: 8EHQ-1001-14915. November 5, 2001. Available online at http://www.epa.gov/opptintr/tsca8e/doc/8esub/8e101501.htm.
[22] Olsen, GW., Burris, JM., Lundberg, JK., Hansen, KJ., Mandel, JH and Zobel, LR (2002). Final Report: Identification of fluorochemicals in human sera. III. Pediatric participants in a group A streptococci clinical trial investigation, Study conducted by Corporate Occupational Medicine, Medical Department, 3M Company, 220-3W-05, St. Paul, MN.
[23] Burris, JM., Olsen, G., Simpson, C and Mandel, J (2000). Determination of serum half-lives of several fluorochemicals: Interim Report #1. Study Sponsor: 3M Company, Corporate Occupational Medicine Department, US EPA AR226-0611.
[24] 3M. 2002. Analysis of pooled human sera and plasma and monkey sera for fluorocarbons using exygen method ExM-023-071. Report prepared for 3M, St. Paul, MN by Exygen Research, State College, PA. Sponsor study number E02-1071; Exygen Study Number: 023-082 U.S. EPA Administrative Record AR226-1152.
PFCs Last Forever
April 2003
3M: PFOA is “...completely resistant to biodegradation”
EPA: “PFOA is persistent in the environment. It does not hydrolyse, photolyse or biodegrade under environmental conditions.” [Extract | Full Document]
Speed is of the essence in the ongoing government reviews. Every new molecule of PFOA produced by the chemical industry in the coming years will be with us forever. PFOA never breaks down.
Even if PFOA were banned today, the global mass of PFOA would continue to rise, and concentrations of PFOA in human blood could continue to build. Long after PFOA is banned, other PFC chemicals from 50 years of consumer products will continue to break down into their terminal PFOA end product, in the environment and in the human body.
Between 1973 and 1988 PCBs, DDT, and related chemicals were banned from use in the U.S. and abroad when it was discovered that their persistence and toxicity, combined with their ability to build up in the food chain and in people, were wreaking havoc on the environment.
Even as these notorious compounds were very publicly phased out and replaced by safer substances, studies performed on perfluorinated chemicals like PFOA were indicating that they also were persistent and bioaccumulative. As these studies were completed, PFC products were quickly making their way into every household in the U.S. in the form of 3M's Scotchgard and Scotchban products, DuPont's Stainmaster and Zonyl products, and DuPont's Teflon.
Industry scientists knew as far back as 1976 that PFCs like PFOA would resist breaking down in the environment. In a technical report summary of biodegradation tests, 3M scientists expound on the persistence of their chemicals in the environment:
“A vast array of organic compounds can be completely degraded by microorganisms. So vast in fact that it was once believed by some that given enough time and the proper conditions, microorganisms could degrade any organic material. This doctrine of microbial infallibility is still a common misconception...
Perfluorinated compounds are extremely resistant to biodegradation... Although compounds with single fluorines have been shown to release fluoride ions as a result of biodegradation, perfluorinated compounds have rarely or never been shown to undergo natural degradation. For this reason, no modification of the perfluoro components of compounds in this study was anticipated.” [1]).
In 1978 PFOA was confirmed to be “completely resistant to biodegradation” in a study done by 3M to substantiate a similar finding in a 1972 National Academy of Sciences report [Degradation of Synthetic Organic Molecules in the Biosphere, NAS 1972). (FC-95 and FC-143).] The primary finding of the new 3M study — “...the results of this study suggest that these chemicals are likely to persist in the environment for extended periods unaltered by microbial catabolism” [1] [Extract | Full Document]. Subsequent studies showed that PFOA in water does not break down, either via the energy of sunlight or through reactions with water itself, processes called aqueous photolysis and hydrolysis. [2, 3]
Collectively, these studies show that PFOA does not break down by any known environmental degradation mechanism: hydrolysis, photolysis, or biodegradation. It is also not broken down in the human or animal gut, but remains unchanged in the body, and stays for years once it finds its way in.

Unlike other persistent organic pollutants, all of which have some capacity to breakdown in the environment, PFOA will persist indefinitely even if banned, and will continually redistribute throughout the environment, the food chain, and the human population. PCBs and DDT have declined in total global mass in the decades following their respective bans in many countries, but the same will not be true for PFOA.
Persistent Organic Pollutants
3M touts perfluorinated compounds on its website:
"It's a good guess that somewhere in the world each day there is someone giving thanks for the powers of Scotchgard protection, that groundbreaking, invisible wonder created by scientists at 3M nearly 50 years ago." [4]
3M's own studies indicated that their "invisible wonder" chemicals were more persistent than DDT, PCB's and Dieldrin. In the late 1970s biodegradation studies performed by 3M indicated that PFOS and PFOA would not biodegrade in activated sewage sludge, a medium rich in microbes and used in standard indicator tests to define upper-bound rates at which bacteria can break down chemicals.
DDT has a reported half-life of seven hours (the time required for half the original mass of chemical to break down) in activated sewage sludge [5], and a half-life of up to 15 years in soil field plots [5]. Up to 66 percent of the commercial PCB mixture called Aroclor 1242 degraded after 28 days of exposure to activated sewage sludge. [6] Dieldrin has been reported to have a half-life of seven years in soil field plots. [7] In contrast, studies performed by 3M in 1976 and 1978 showed that PFOA, PFOS, and other terminal breakdown products of PFC products do not degrade at all, even in activated sewage sludge, giving them an infinite half-life.
Some PFCs break down, but the breakdown chain stops at PFOA, PFOS and other terminal products. Later studies indicated that some PFCs related to PFOS were capable of undergoing biodegradation, but analysis of the biodegradation products revealed that these chemicals decompose to terminal, non-biodegradable PFOS and PFOA [9] (Figure 1).
Figure 1. [Excerpt | Full Document]
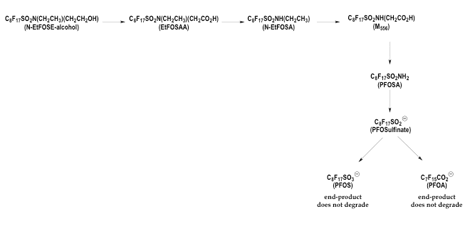
Fluorotelomers break down into PFOA, which never breaks down. Of the fluorotelomers, used in Stainmaster and Zonyl paper and fabric protectors, DuPont's Korzeniowski is quoted in the April 12, 2001 edition of Environmental Science and Technology, as saying:
"PFOS seems to behave differently from our products [fluorotelomers]." and "Scientific information and studies on these materials are too limited to say whether they break down or not." [10]
But two separate studies show that fluorotelomers are not so different from the 3M compounds, in fact in some cases after biodegradation they are the same compounds, one published in the peer-reviewed literature in 1981, and another recently sponsored by 3M. In both of these studies, scientists found that biodegradation of the fluoroteolomers results in PFOA and other chemicals in the PFOA family with differing carbon chain lengths (the perfluorinated carboxylic acids).
For example, 3M found that after the Zonyl BA-type mixture of telomer alcohols was exposed to activated sewage sludge for 16 days, the mixture of fluorotelomers had largely decomposed to perfluorinated carboxylic acids containing between 5 and 12 carbon atoms [11] [Extract | Full Document]. Degradation of the longer chain fluorotelomers (16 carbons in length) was too slow to measure.
Scientists at Pace Analytical Services explain that each fluorotelomer alcohol will biodegrade to two perfluorinated carboxylic acids (PFOA and related chemicals), an acid containing one fewer carbon than the initial telomer, and an acid containing two fewer carbons than the initial telomer alcohol (Figure 2). [11] The fluorotelomer alcohol with a ten-carbon chain breaks down predominantly to PFOA.
Figure 2.

Degradation by hydroxyl radicals in the upper atmosphere (the troposphere) is another important means of environmental degradation. United Nations Environmental Program guidelines suggest that a compound with an atmospheric half-life of two days or five days or more should be considered persistent [12]; this is because atmospheric transportation is such a rapid method of molecule transport.
DDT, the PCB mixture Aroclor 1242, and Dieldrin have been reported to undergo decomposition by hydroxyl radicals with half lives of 5 days, 4.6 to 98 days, and 42 hours respectively [13]. POSF (a semivolatile precursor of PFOS), on the other hand, is proposed to undergo degradation with a half-life of greater than 3.7 years, if it occurs at all (in six of seven experiments, researchers observed no degradation of POSF) [14]. This greatly exceeds the UNEP recommendation and provides more evidence that the perfluorinated compounds are more persistent than the chlorinated compounds that raised such great concern in the 1970s. And PFOS and PFOA are not believed to degrade at all by this mechanism.
Studies indicate that PFOA and PFOS are also resistant to hydrolysis, another major means of environmental degradation, and although many PFOS derivatives are expected to undergo slow hydrolysis (N-EtFOSE-alcohol has an estimated hydrolysis half-life of 6.3 years or more than 27 years, in two studies), PFOS is the ultimate product of hydrolysis.
Slow breakdown rates of consumer product chemicals to PFOS and PFOA is a concern. EPA forced 3M to phase out use of its Scotchgard PFOS chemicals in May of 2000 because of concerns about current levels of PFOS in human blood in relation to levels shown to harm lab animals. EPA’s regulatory posture on PFOA also stems from concerns over current levels in humans. In both these cases, even if a total global production ban were in place, concentrations might continue to rise in human blood as the chemicals in consumer products (Stainmaster telomer alcohols, the original Scotchgard ingredients, and others), slowly break down in the environment over several decades to their terminal degradation products — PFOS and PFOA.
Some PFCs can build up in the food chain, to concentrate in humans.
Although early bioconcentration studies on PFCs were not without errors, they did indicate that PFCs would have the potential to build up in the food chain. In 1979, 3M’s Environmental Laboratory found that fish exposed to effluent from the Company's Decatur, Alabama fluorochemicals plant had significant concentrations of PFOS and Scotchgard’s raw ingredient N-Et PFOSE-alcohol. The authors concluded that these compounds build up, or bioconcentrate, in fish.
In 2003, in research supported by Health Canada and Environment Canada, Martin and coworkers determined that some PFCs could build up in the food chain to the same extent as PCBs [15], which despite their having been banned more than a quarter of a century ago, continue to render freshwater fish unsafe to eat in waters across 38 states [16].
PFOA is known to contaminate the food chain, including wild fish, other wildlife, and food on grocery store shelves [Extract | Full Document], but Martin’s study shows that other PFCs have a much greater potential to pollute the food supply. The bioconcentration factors (BCFs) reported for some of the longer-chain compounds in the PFOA family are equivalent to those for PCBs. These chemicals, like PFOA, are breakdown products of fabric and paper protection formulations (Table 1).
Table 1.
|
Chemical |
Source in the environment |
Bioconcentration Factor (potential to contaminate the food chain and concentrate in humans) |
Known food supply contamination |
|
PFOA (Teflon, Stainmaster) |
Pollution from Teflon plants, offgas from Teflon, breakdown product of Stainmaster and other PFC products |
4.0 ± 0.6 |
Found in produce, meat, and bread in grocery stores |
|
PFDA (10-carbon version of PFOA) |
Breakdown product of Stainmaster and other PFC products |
450 ± 62 |
No tests available |
|
PFOS (Scotchgard) |
Breakdown product of 3M’s old Scotchgard formulation (pre-2000) |
1,100 ± 150 |
Found in [list products] |
|
PCBs (Aroclor 1242) |
Banned chemicals, formerly used in electrical capacitors and other industrial applications |
1,000 to 25,900 |
Widely contaminates meat and dairy products around the world |
|
PFUnA (11-carbon version of PFOA) |
Breakdown product of Stainmaster and other PFC products |
2,700 ± 400 |
No tests available |
|
PFDoA (12-carbon version of PFOA) |
Breakdown product of Stainmaster and other PFC products |
18,000 ± 2700 |
No tests available |
|
PFTA(14-carbon version of PFOA) |
Breakdown product of Stainmaster and other PFC products |
23,000 ± 5300 |
No tests available |
Source: Environmental Working Group compilation of BCF data in the peer-reviewed literature.
References:
- 3M. 2000. Biodegradation study of PFOS. US Environmental Protection Agency Administrative Record Number AR226-0057.
- 3M. 2001. Screening Studies in the Aqueous Photolytic Degradation of Perfluorooctanoic Acid (PFOA). U.S. EPA Administrative Record AR226-1030 Photolysis E00-2192.
- 3M. 2001. Hydrolysis Reactions of Perfluorooctanoic Acid (PFOA). U.S. EPA Administrative Record AR226-1030a090.
- 3M. About 3M. available online at www.3m.com/about3m/innovation/inventors_hall/index.jhtml.
- HSDB. DDT. available online at http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB.
- HSDB, rciHTH. 1981. J Water Pollut Contr Fed 53: 1503-18.
- Institute, HRcwHCIaT. 1992. Biodegradation and Bioaccumulation Data of Existing Chemicals Based on the CSCLJapan, Japan Chemical Industry Ecology — Toxicology and Information Center.
- Company, M. 1976. Biodegradation Studies of Fluorocarbons. U.S. EPA Administrative Record AR226-0356.
- 3M. 2001. Executive Summary of Biodegradation Studies. U.S. EPA Administrative Record AR226-1030a107.
- Renner, R. 2001. Growing Concern over Perfluorinated Chemicals. Environ. Sci. and Technol.: 154A-160A.
- Services, PA. 2002. Biodegradation Study Report: Biodegradation Screen Study for Biodegradation Screen Study for Telomer Type Alcohols,. U.S. EPA Administrative Record AR226-1149.
- UNEP. 1999. available online at www.chem.unep.ch/pops/POPs_Inc/INC_2/en/infs/inc2_inf2.htm.
- Syracuse Research Corporation., Meylan W.M. and Howard P.H. 1993. Chemosphere 26(as cited in the HSDB): 2293-2299.
- 3M. 2001. Indirect Photolysis of Gaseous Perfluorooctane Sulfonyl Fluoride (POSF) by Fourier Transform Infrared (FTIR) Spectroscopy. U.S. EPA Administrative Record AR226-1030a104.
- Martin, JW., Mabury, SA., Solomon, KR and Muir, DC. 2003. Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 22(1): 196-204.
- EPA), UEPAU. 2002. Update: National Listing of Fish and Wildlife Advisories. available online at: http://www.epa.gov/waterscience/fish/advisories/factsheet.pdf.
- US Environmental Protection Agency (US EPA) (2001). Analysis of PFOS, FOSA, and PFOA from various food matrices using HPLC electrospray/mass spectrometry, 3M study conducted by Centre Analytical Laboratories, Inc.
Notes relating to graph
Assumptions
- Assumed environmental halflife for C-10 fluorotelomer alcohol: 8.6 years. We find no studies that measure degradation of C-10 under normal environmental conditions. We estimated an environmental halflife for the C-10 fluorotelomer alcohol by scaling the environmental halflife for DDT (10.9 years, detailed in assumption #4) by the ratio of halflives of the alcohol and DDT in activated sewage sludge (where DDT degrades by 50 percent in seven hours, according to Johnson (1976), and where 95% of C-10 degrades in 24 hours (Pace 2002)).
- Increases in PFOA are calculated based on predicted breakdown rates of C-10 fluorotelomer alcohol, assuming that 12/13ths of the mass of the alcohol converts to PFOA, consistent with Pace (2002).
- Implicit in this figure is the assumption that the total moles of C-10 fluorotelomer alcohol and PFOA in the environment, at the time PFOA is banned, are equal.
- Assumed environmental halflife for DDT: 10.9 years, based on observed 81 percent decline in breast milk in Germany between 1969 and 1995 (Solomon and Weiss 2002).
References (graph)
- Johnson RE. 1976. Res Rev 61:1-28.
- Pace Analytical Services. 2002. Biodegradation Study Report: Biodegradation Screen Study for Biodegradation Screen Study for Telomer Type Alcohols. U.S. EPA Administrative Record AR226-1149.
- Solomon GM and PM Weiss. 2002. Chemical contaminants in breast milk: time trends and regional variability. Environmental Health Perspectives. 110(6), A339-447.
PFC Health Concerns
April 2003
In new laboratory work scientists find that low doses of PFOA harm lab animals — at estimated blood levels lower than those found in some children. The government initiated in-depth analyses of human risk on receiving 3M lab studies in May 2001. And now, with calculated risks to human health far too high, the government is poised to demand rare, expedited assessments.
Industry’s most recent study shows organ weight changes — often a gross sign of toxicity and damage to organ function — among lab animals exposed to PFOA in the womb and into early adulthood. [Organ weight effects in: males | females] [Mortality and sexual development: Extract | Full document] Some human children and adults have more PFOA in their blood than the estimated levels for the animals in this study. Under duress from the Environmental Protection Agency, 3M and DuPont are handing over unprecedented amounts of health and safety data — some 50,000 pages worth to date.
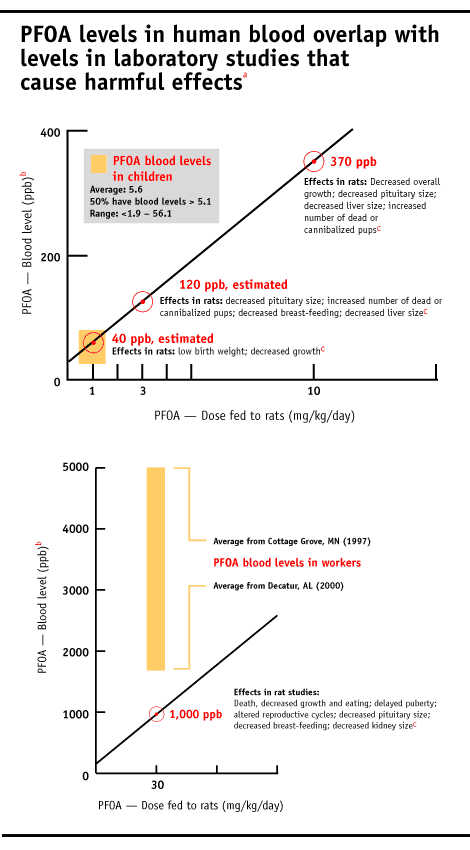
Three of the four tumors caused by PFOA are on the rise in people, including testicular, breast, liver and prostate. [Extract] PFOA also causes hypothyroidism in lab studies, a condition linked to fetal brain damage. Hypothyroidism strikes 4.6% percent of Americans, mostly women [1]. Levels of PFOA in some people’s bodies now appear to be in the range known to harm animals.
EPA’s new findings of high risk to humans. EPA instigated its in-depth analysis of human risk from PFOA exposures on receipt of a major new study, sponsored by 3M, in 2002. In this rat reproduction study scientists exposed rats to PFOA in utero through early adulthood, and found damage to organs in animals exposed to the lowest doses tested [2]. [Reproductive effects in: males | females]
At the lowest dose tested, with levels of PFOA in the maternal blood of approximately 40 parts per billion (ppb), the offspring were smaller at birth and the adult female rats exposed in utero (F1 generation females) decreased body weight gain at certain times in young adulthood [2] [Extracts: F1 newborn, F1 adult body weight | Full document Note: 22MB file]. At three of four doses tested, beginning with maternal blood at approximately 120 ppb, the adult F1 generation female rats had decreased growth of the pituitary gland [2] [Excerpt | Full document Note: 22MB file]. The pituitary gland is often called the “master gland” in the body because it secretes hormone that regulate many bodily processes, such as growth, maternal care, reproduction and metabolism. When the adult F1 female rats gave birth to their own babies (called the F2 generation), a greater number of the F2 pups were “found dead or presumed cannibalized” [2] [Extract | Full document Note: 22MB file]. This suggests that maternal care could have been altered, perhaps by damage to the pituitary gland. Alternatively, the F1 mothers may have ignored or cannibalized pups because the F2 offspring were not healthy.
At higher doses in the rat reproduction study, corresponding to 1 part per million (ppm) PFOA in maternal blood, seven of 60 male and six of 60 female offspring died [Reference to Extract | Full document Note: 22MB file]. No excess mortality was found in parental animals [Extract], which means that PFOA is more toxic to the young compared to adults. As a group, the male offspring were emaciated, cold to touch and less active than untreated rats. When scientists autopsied the male and female offspring, they found significant changes in eight organs, including the brain, liver, spleen, thymus, adrenal gland, kidney, prostate, testes and epididymides. Both male and female offspring reached sexual maturity later and the female offspring also had altered reproductive cycling.
3M workers involved with PFOA production typically have blood PFOA levels above 1 ppm, and as high as 114 ppm in one worker tested. DuPont does not readily disclose worker PFOA blood levels, although a document made public in recent litigation, marked “Personal and Confidential” shows that two of eight women tested at the Washington Works plant in 1981 had PFOA blood levels higher than 1 ppm. [Full Document] A recently publicized internal DuPont hazard assessment shows that people who drink water with 3 ppb PFOA for six years are expected to accumulate blood levels of 1 ppm.[Extract and EWG analysis] PFOA is found at this level in drinking water supplies near DuPont manufacturing facilities.
EPA classifies PFOA as carcinogenic in animals, causing testicular, pancreatic, mammary and liver tumors in rats [3]. Workers exposed to PFOA have elevated risks of dying from or seeking treatment for cancers of the pancreas and male reproductive tract, including those of the testis and prostate.[EWG Worker Study Document] Testicular, breast, and liver cancer have been increasing in the US during the past 10 to 25 years.[Extract] Liver cancer alone has increased an estimated average 4.7% a year between 1992 and 1999 [4].
Five studies have shown that PFOA alters reproductive hormones in the male, causing increased levels of estrogen and abnormal testosterone regulation [5-9] Increased levels of estrogen have been found in exposed workers [10, 11] [Extract | Full document]. In rats, PFOA alters growth of the prostate, testis, epididymides, and seminal vesicles.
Eleven studies show that PFOA or chemicals that break down into PFOA damage the thyroid gland. In 2002, monkeys exposed to PFOA for one month developed an underactive thyroid, a condition called hypothyroidism.[Extract] Hypothyroidism affects an estimated 4.6 percent of Americans [1], mostly women. Hypothyroidism can impair brain development, leading to hearing loss and impaired growth and intellectual development.
Four organs or tissues in the immune system and at least nine types of cells that regulate immune function are targets of PFOA [34-37]. Thus far, scientists have failed to find a dose of PFOA that does not damage the immune system.
So far, five different pathways have been identified that might explain how PFOA causes cancer and other types of toxicity. These include mitochondrial toxicity; cell membrane disruption that results in decreased cell communication; peroxisome proliferation; increased levels of estrogen and decreased levels of testosterone; and decreased thyroid hormone levels.
Cancer.
The federal government considers PFOA to be carcinogenic — causing liver, pancreatic, testicular, and mammary gland tumors in rats [3] [p. 6]. Three of these four cancers have been increasing in the US population in recent years. Breast cancer strikes one in eight women. The incidence of testicular cancer has risen in certain parts of the world during the last several decades and is now the most common type of cancer in men aged 15 to 35 [12].
In two-year cancer studies sponsored by 3M and DuPont, none of the 80 rats in the “control group” developed testicular or pancreatic tumors; in contrast, these tumors were found in eight of 76 (11%) exposed to PFOA [3, 9 pg. 75]. In a two-year cancer study conducted by 3M, PFOA doubled the incidence of mammary tumors in exposed laboratory animals [13].
3M has seen problems with these kinds of cancers among their workers as well. In various studies of their workers’ health, 3M reported increased rates of dying or seeking care for prostate cancer, testicular cancer, and pancreatic cancer or disease [14-16].[EWG Worker Study Document] These worker studies typically involve so few people that the increases are often considered to be statistically weak. Nevertheless, the consistency of cancers among workers and in laboratory studies is striking. While a cause and effect link between human cancers and PFOA exposures has not been established, the increases in these cancers, combined with ubiquitous PFOA contamination in human blood is cause for concern.
Breast cancer.
Among girls born today, one in eight is expected to get breast cancer and one in 32 is expected to die from it [4]. Breast cancer in women increased an average of 1.1 percent per year between 1992 and 1999 [4]. Among those 65 and younger, breast cancer incidence rose 1.4 percent per year [4]. If these trends continue, the granddaughters of today's young women could face a one-in-four chance of developing breast cancer [17, 18].
Testicular cancer.
At its current pace, the incidence of testicular cancer is doubling about every one and a half generations (39 years). In the U.S. the incidence of testicular cancer rose 41.5 percent between 1973 and 1996, an average of 1.8 percent per year [17, 18]. While rates of testicular cancer continue to drop among older men (65 and up), testicular cancer remains the most common cancer among young men, with the highest rate of diagnosis among men between the ages of 30 and 34.
Prostate cancer.
Prostate cancer rates rose 4.4 percent a year between 1973 and 1992, or more than a doubling of risk in a generation. Since 1992, the incidence has declined, but it is still 2.5 times the 1973 rate. Part of this increase can be explained by better detection, but increased incidence has also been accompanied by an increase in mortality — which better detection cannot explain. Prostate cancer is now the most common cancer among U.S. men, and the second most lethal [17, 18].
Worker studies show increased rates of developing and dying of certain cancers.
3M workers exposed to high levels of fluorochemicals like PFOA appear to be at higher risks for cancers of the male reproductive system [14, 15].[EWG Worker Study Document] Mortality studies of 3M workers at the Cottage Groove, MN plant found that Chemical Division workers with ten or more years of employment were 3.3 times more likely to die of prostate cancer compared to workers who did not work in PFOA production [14].[Extract] In two other studies, one conducted in Cottage Groove, MN and the other at a 3M plant in Decatur, Alabama, 3M found that exposed workers had elevated risk for dying of prostate cancer or visiting the doctor for reasons associated with having prostate cancer [15, 16]. While prostate cancer is fairly common among older men — one in 5 or 6 will develop the disease — only about one in 30 will die from prostate cancer and 50% of men with prostate cancer will die after the age of 79 [4]. 3M chose not to study cancer incidence among workers, but instead studied cause of death. The average age of death in men working in the chemical division of 3M was 54.2 years [14].
While these two studies did not report statistically elevated risk like Gilliland et al. did, the findings are consistent across the worker studies and also with animal studies showing that the prostate is a target organ of PFOA [2]. Workers in the two 3M plants are also more likely to die or seek treatment for pancreatic cancer or disease and any type of male reproductive tract cancer, which includes testicular and prostate cancer [15, 16]. Neither pancreatic or testicular cancers are as common in men as prostate cancer, and the likelihood of dying from these diseases is not high: the lifetime risk for dying of pancreatic cancer is about one in 87, and for testicular cancer about 1 in 5000 [4]. Again, 3M studied cause of death and not disease incidence. Because dying from these types of cancer is not common, it is all the more troubling that increased risks were noted for these diseases. If PFOA is causing these effects in workers, a much larger study than the studies conducted by 3M would be needed to find statistically significant effects. Nevertheless, the patterns of disease are remarkably consistent with animal studies.
All of the worker studies conducted by 3M and DuPont have significant flaws that prevent conclusive interpretation of study results. The flaws in the worker studies would tend to obscure the ability of scientists to discern exposure-driven health effects, making the many findings of health harms in various worker studies particularly compelling. For example, in some studies 3M classified workers into exposed or unexposed categories based on job occupation to see if there were differences in diseases between these workers [14-16, 19]. Yet, in 1996, authors of a study partially sponsored by 3M concluded that PFOA contamination among all workers was so ubiquitous that job history could not be used as a measure for exposure:
We expected the group of workers who were selected for the unexposed group based on job history to have total serum fluorine levels similar to the general population. However, we found that this group of workers was not unexposed, having levels 20-50 times higher than levels reported for the general population. We concluded that job history was not an accurate metric for exposure.
Hypothyroidism.
In eleven studies conducted between 1978 and 2002, scientists have documented damage to the thyroid gland following exposure to PFOA and chemicals that break down into PFOA, in monkeys and other animals [13, 20-26]. The damage includes cellular effects on the thyroid and hypothyroidism, a condition characterized by low levels of thyroid hormones that control growth and metabolism and that are critical for proper brain development.
Thyroid cancer and hypothyroidism are current human health concerns. An estimated 10 million people in the US have hypothyroidism [27]. The condition is of particular concern in pregnant women because thyroid hormones are critical for proper brain development in the fetus. Small reductions in maternal thyroid hormone levels during pregnancy have been associated with reduced IQs in children.
In a 1998 study at the Cottage Groove, MN plant, 3M found evidence of altered thyroid hormone regulation in workers. Medical staff measured significant increases in thyroid stimulating hormone (TSH) in workers with higher PFOA blood levels [10, 11].[TSH Findings: 1998 Study | DuPont Summary of TSH | Dupont Hazard Assessment] High TSH levels are one sign of an underactive thyroid and resulting low thyroid hormone levels; the pituitary gland in the brain will secretes extra TSH as a signal to stimulate thyroid hormone production.
Industry scientists found a trend towards decreased thyroid hormone levels in every group of PFOA-exposed animals in a 2002 monkey study, [20] [Extract] and increased cellular damage in the thyroids of rats exposed to chemicals that break down into PFOA [20-26]. The cellular changes are the same type caused by a prolonged state of hypothyroidism and are also associated with developing thyroid tumors in rodents [29, 30]. DuPont has never tested whether the chemicals that break down into PFOA cause tumors.
Three new studies show that compounds that break down into PFOA in the body also target the thyroid. Although detailed study information is claimed as CBI (Confidential Business Information) and redacted from the public record [23] [Extract], presentations made by DuPont to the EPA indicate that the thyroid was a target for all of the fluorinated telomers tested, including those known to break down into PFOA [25] [Extract | Full document]. DuPont interpreted these effects as “non-adverse physiological responses,” but under their claim of “CBI” privilege provided no information on the specific types of effects seen [23].
An underactive thyroid gland in adults can lead to fatigue, depression, anxiety, unexplained weight gain, hair loss, and low libido. More serious, however, are the effects of thyroid hormone disruption for the developing fetus and child. Fetuses, infants and children who experience more significant changes in hormone levels may suffer mental retardation, loss of hearing and speech, abnormal testicular development or deficits in motor skills. In older children, depressed thyroid levels have been associated with lower motivation to learn and attention deficit disorder [31, 32].
Industry attempts to obscure and minimize thyroid findings. 3M and DuPont continue to downplay the effects of PFCs on the thyroid. In a study co-authored mostly by industry scientists, the researchers state that “there were no APFO [PFOA]-related changes in clinical chemistry, hormones, or urinalysis, or hematological effects” in monkeys. Yet later in the article, the scientists include a table showing that PFOA exposures led to significantly decreased thyroid hormone levels, and the authors report that thyroid hormones became more normal after PFOA exposure stopped [20].[Extract]
Similarly, in 1998, 3M scientists published a study that included a table showing that workers with high blood levels of PFOA had statistically significant increases in TSH, a measure of hypothyroidism [11] [Extract]. Yet, they failed to discuss this finding in the study summary, results or discussion section.
DuPont recently presented data to the EPA showing that their fluorotelomer compounds affect the thyroid, but interpreted these effects as “non-adverse physiological responses,” and provided no specific details of the thyroid effects, citing this data as “Confidential Business Information” [23].
3M recently undertook a comprehensive assessment of the effects of PFOA to the fetus. Inexplicably, among the many health endpoints studied, they neglected to assess the thyroid at all [2], even though PFCs are so clearly linked to thyroid damage in other studies, and PFOA has been found to cause hypothyroidism in monkeys.
Immune system problems.
In laboratory studies PFOA causes toxicity to four organs or tissues in the immune system and at least nine types of cells that regulate immune function [2, 33, 37]. PFOA has long been known to damage the immune system, but in the most recent study scientists learned that exposures to PFOA early in life are more harmful than in adulthood. In this study scientists failed to find a dose that did not damage the immune system. The spleen and thymus, both critical to immune function, were atrophied among animals exposed in the womb and through early adulthood; spleen atrophy occurred at the lowest dose tested.
In industry’s latest animal study, every dose tested harmed the spleen, a gland where specialized cells called B cells mature and then produce antibodies critical to combating disease. The thymus gland seems particularly sensitive to chemicals in the PFC family. Lodged behind the breastbone and above the heart in humans, the gland plays a critical role in immunity, manufacturing T cells that recognize and destroy bacteria, viruses, and cancer cells. At least one PFC chemical (PFDA) causes the gland to rot away to the point where scientists can no longer find it in the animal: “Thymic tissue was not found in the majority of treated rats.” [38][Extract] While PFOA has not been shown to cause the thymus to completely disappear, it has been linked to atrophy of the gland in animals dosed in the womb and through early life, at doses that cause no thymus effects in animals exposed only during adulthood [2].
In a 1995 symposium on early-life immune impacts, a consensus statement from a diverse panel of scientists implies the significance of immune system damage in early life: “Life-long capacity for immune response is determined early in development, during prenatal and early postnatal development in mammals” [39]. Many immunotoxic chemicals produce more severe or long-lasting damage when exposure occurs early in life [40]. In the fetus and through early life, the thymus is instrumental in fostering the growth and development of the immune system. The type of thymic damage observed with PFOA could lead to permanent decrements in immune function, resulting in higher risk for infection and disease, including cancer.
Several studies by scientists in labs at Stockholm University and the Karolinska Institute in Sweden looked at the effects of PFOA on immune cells in detail. They found that PFOA decreased the number of every immune cell subpopulation they studied — eight in all — in the thymus and spleen [35, 37]. Yang et al. also found PFOA damaged immune cell function, a phenomenon as the cells were unable to mount a proper immune response to foreign cells, referred to as immunosupression [34].
A few of the effects of PFOA on immune system cells are due to activation of a receptor (peroxisome proliferating activating receptor-alpha or PPARa) that mediates several other toxic effects of PFOA and that is thought to be a more active mechanism in rodents than in humans. When scientists feed PFOA to mice in which the capacity for this mechanism has been genetically removed, many of the effects on the thymus cells remain, reinforcing the relevance of the laboratory studies for humans, and heightening the concern for in utero and early life human exposures to PFOA.
In workers, increased blood levels of PFOA are associated with increased white blood cells (leucocytes) [10], suggesting that workers are under stress from infection or disease [41], consistent with a picture of poor immune function.
Reproductive problems, birth defects.
PFOA is more toxic to fetuses and infants than to adult animals. For example, PFOA causes death in young rats at doses that do not affect survival in the parents.
Much of the EPA’s concern for PFOA stems from the results of a 2002 rat reproduction study paid for by 3M [2]. In this study, adult rats were dosed with PFOA prior to mating, during mating and pregnancy, and throughout lactation until their offspring are weaned at about 3 weeks of life. The offspring were further dosed with PFOA and allowed to breed. In this way, the EPA can see whether PFOA decreases fertility, as well as decide if PFOA exposure early in life causes developmental toxicity.
The rat reproduction study showed was that PFOA is more toxic to young animals [2]. Rats exposed to PFOA in the womb often died at weaning in the highest dose group even though mortality was not affected in adult rats at any dose level. Also, a greater number of organs were affected by PFOA in adult male rats exposed in utero at the lowest PFOA dose compared with adult male rats not exposed during fetal life.
In male rats exposed only during adulthood, two organ weights (liver and kidney) were increased at the lowest dose. Four organ weights were altered in adult males exposed to PFOA in the womb; animals in this group tended to have decreased body weight and had significantly increased liver, kidney, and seminal weights and decreased spleen weight. Organ weight changes are used as a gross measure of toxicity and often indicate impared organ function.
A 1982 study sponsored by 3M also showed that PFOA is more toxic to rabbits exposed in the womb than in adulthood [42]. In this study, rabbit fetuses had significantly increased number of skeletal abnormalities at a dose that did not cause effects on the mother.
DuPont tested for and found PFOA in the blood of female plant workers in Parkersburg. The company followed and documented pregnancy outcomes in exposed workers. Two of seven children born to female plant workers between 1979 and 1981 had birth defects, one an “unconfirmed” eye and tear duct defect, and one a nostril and eye defect.[Full Document] In 1981 fifty women were reassigned in the plant.
In addition to causing testicular tumors, PFOA causes many other effects on the male reproductive system, including increased size of the testes, epididymides and seminal vesicles[2], and decreased prostate in rats [2, 6]. In the female, PFOA causes mammary tumors and cellular effects on the ovary [13].
Beginning in 1992, DuPont scientists began to publish papers addressing how PFOA causes testicular tumors and other harmful effects on the male reproductive tract (they have not studied mammary gland and ovarian effects). First, they found that PFOA increases blood levels of estradiol (the major form of estrogen in humans and rodents) in male rats. They also found that PFOA affects testosterone regulation, tending to decrease blood levels of testosterone and alter the production of testosterone in testicular cells[5], effects that are likely due to a “lesion at the level of the testes” [10].
A follow-up study published by DuPont scientists in 1995 showed that PFOA increases levels of estrogen by increasing activity of liver aromatase, an enzyme that converts testosterone to estradiol [5]. Biegel et al. also found that PFOA increased testicular levels of a protein produced in high levels by cancer cells called transforming growth factor-alpha (TGFa) [5]. While DuPont scientists have not studied female rats as often as male rats, other studies have shown that estradiol stimulates excess release of TFG-a in mammary cells.
Because high levels of estrogen are a risk factor for the type of testicular tumor caused by PFOA, EPA suggested that the induction of Leydig cell tumors, a type of testicular tumor, by PFOA may be endocrine mediated, possibly by sustained elevation of estrogen [6].[Extract | Full Document]
Increased estradiol and decreased testosterone have been found in highly exposed 3M workers at a plant that produced PFOA in Cottage Groove, MN. [10, 11]. Three studies in two 3M plants have confirmed that exposed workers appear more likely to die or seek treatment for cancers of the male reproductive tract [14-16].
References:
[1] Hollowell, JG., Staehling, NW., Flanders, WD., Hannon, WH., Gunter, EW., Spencer, CA and Braverman, LE. 2002. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87(2): 489-99.
[2] York, RG (2002). Oral (gavage) two-generation (one litter per generation) reproduction study of ammonium perfluorooctanoate (APFO) in rats. Report prepared for 3M, St. Paul, MN by Argus Research (Horsham, PA). Sponsor's Study No. T-6889.6., Reviewed in US EPA AR226-1092.
[3] Environmental Protection Agency (EPA). 2002. Revised draft hazard assessment of perfluorooctanoic acid and its salts, November 4, 2002. U.S. EPA Administrative Record AR226-1136.
[4] Ries, LAG., Eisner, MP., Kosary, CL., Hankey, BF., Miller, BA., Clegg, L and Edwards, BK. 2002. SEER Cancer Statistics Review 1973-1999: Overview in a Single PDF. National Cancer Institute. Bethesda, MD. Available online at http://seer.cancer.gov/csr/1973_1999/sections.html.
[5] Biegel, LB., Liu, RC., Hurtt, ME and Cook, JC. 1995. Effects of ammonium perfluorooctanoate on Leydig cell function: in vitro, in vivo, and ex vivo studies. Toxicol Appl Pharmacol 134(1): 18-25.
[6] Cook, JC., Murray, SM., Frame, SR and Hurtt, ME. 1992. Induction of Leydig cell adenomas by ammonium perfluorooctanoate: a possible endocrine-related mechanism. Toxicol Appl Pharmacol 113(2): 209-17.
[7] Liu, RC., Hahn, C and Hurtt, ME. 1996. The direct effect of hepatic peroxisome proliferators on rat Leydig cell function in vitro. Fundam Appl Toxicol 30(1): 102-8.
[8] Liu, RC., Hurtt, ME., Cook, JC and Biegel, LB. 1996. Effect of the peroxisome proliferator, ammonium perfluorooctanoate (C8), on hepatic aromatase activity in adult male Crl:CD BR (CD) rats. Fundam Appl Toxicol 30(2): 220-8.
[9] Biegel, LB., Hurtt, ME., Frame, SR., O'Connor, JC and Cook, JC. 2001. Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats. Toxicol Sci 60(1): 44-55.
[10] DuPont (1997). Hazard characterization for human health C8 exposure CAS registry no. 3825-26-1. Prepared by L.B. Biegel, Senior Research Toxicologist.
[11] Olsen, GW., Gilliland, FD., Burlew, MM., Burris, JM., Mandel, JS and Mandel, JH. 1998. An epidemiologic investigation of reproductive hormones in men with occupational exposure to perfluorooctanoic acid. J Occup Environ Med 40(7): 614-22. Also reviewed in U.S. EPA Administrative Record AR226-1137 (pages 147-149; PDF pages 44-46).
[12] NCI (National Cancer Institute). 2000. Testicular Cancer: Questions and Answers. Available online at http://cis.nci.nih.gov/fact/6_34.htm. Accessed January 16, 2003.
[13] Sibinski, LJ. 1987. Two-Year oral (diet) toxicity/carcinogenicity study of fluorochemical FC-143 (perfluorooctane ammonium carboxylate) in rats. Report prepared for 3M, St. Paul, Minnesota by Riker Laboratories Inc. Study No. 0281CR0012; 8EHQ-1087-0394, October 16, 1987 Reviewed in US EPA "Revised Draft PFOA Hazard Assessment-Robust Study Annex" AR226-1137, p. 260-267.
[14] Gilliland, FD and Mandel, JS. 1993. Mortality among employees of a perfluorooctanoic acid production plant. J Occup Med 35(9): 950-4.
[15] Alexander, B (2001). Mortality study of workers employed at the 3M Cottage Grove facility. Final Report. Division of Environmental and Occupational Health, School of Public Health, University of Minnesota, April 26, 2001, Reviewed in U.S. EPA Administrative Record AR226-1137 (page 143-146; PDF page 40-43).
[16] Olsen, GW., Burlew, MM., Hocking, BB., Skratt, JC., Burris, JM and Mandel, JH. 2001. An epidemiologic analysis of episodes of care of 3M Decatur chemical and film plant employees, 1993-1998. Reviewed in US Environmental Protection Agency Administrative Record AR226-1137 (pages 156-159; PDF page 53-56).
[17] National Cancer Institute (NCI). 1996. SEER Cancer Statistics Review. 1973-1996. Available online at http://www.seer.ims.nci.nih.gov/Publications/CSR1973_1996/.
[18] National Cancer Institute (NCI). 1997. SEER Cancer Statistics Review. 1973-1997. Available online at http://www.seer.ims.nci.nih.gov/Publications/CSR1973_1997/.
[19] DuPont. 1978. Personal and confidential: Lab test summaries for Dupont PFOA workers - September 20, 1978.
[20] Butenhoff, J., Costa, G., Elcombe, C., Farrar, D., Hansen, K., Iwai, H., Jung, R., Kennedy, G, Jr.., Lieder, P., Olsen, G and Thomford, P. 2002. Toxicity of Ammonium Perfluorooctanoate in Male Cynomolgus Monkeys after Oral Dosing for 6 Months. Toxicol Sci 69(1): 244-257. Also reviewed in US EPA Reviewed in US EPA "Revised Draft PFOA Hazard Assessment-Robust Study Annex" AR226-1137, p. 244-253.
[21] DuPont Haskell Laboratory. 2002. Developmental and one-generation reproduction study: Mixture of poly(difluoro-methylene), alpha-fluoro-omega [2-(phosphonooxy) ethyl]-, monoammonium salt (CAS# 65530-71-4); poly(difluoro-methylene), alpha-fluoro-omega[2-(phosphonooxy) ethyl]-, diammonium salt (CAS# 65530-72-5); poly(difluoromethylene), alpha, alpha’- [phosphinicobis(oxy-2,1-ethanediyl)bis [omega-fluoro-], ammonium salt (CAS# 65530-70-3); isopropyl alcohol (CAS# 67-63-0); and water (CAS# 7732-18-5). US Environmental Protection Agency: Toxic Substance Control Act (TSCA) Section 8(e) Submission Received from 01/02/03 to 1/15/03: 8EHQ-1202-15247A. December 20, 2002. Available online at http://www.epa.gov/opptintr/tsca8e/doc/new8e.htm.
[22] DuPont Haskell Laboratory. 2002. Subchronic toxicity study: Mixture of poly(difluoro-methylene), alpha-fluoro-omega [2-(phosphonooxy) ethyl]-, monoammonium salt (CAS# 65530-71-4); poly(difluoro-methylene), alpha-fluoro-omega[2-(phosphonooxy) ethyl]-, diammonium salt (CAS# 65530-72-5); poly(difluoromethylene), alpha, alpha’- [phosphinicobis(oxy-2,1-ethanediyl)bis [omega-fluoro-], ammonium salt (CAS# 65530-70-3); isopropyl alcohol (CAS# 67-63-0); and water (CAS# 7732-18-5) (Telomer B Phoshate). US Environmental Protection Agency: Toxic Substance Control Act (TSCA) Section 8(e) Submission Received from 02/27/02 thru 03/13/02: 8EHQ-0202-15072A. February 6, 2002. Available online at http://www.epa.gov/oppt/tsca8e/doc/8esub/8e031302.htm.
[23] DuPont. 2002. The updated copy of DuPont Product Stewardship on December 17, 2001. U.S. EPA Administrative Record AR226-1069.
[24] DuPont Haskell Laboratory. 2002. Results of an oral gavage combined 90-day repeated dose and one-generation reproductive toxicity study in rats for poly (oxy-1,2-ethanediyl) alpha-hydro-omega-hydroxy- ether, with alpha-fluoro- omega (2-hydroxyethyl) poly (difluoromethane) (1:1) (telomer B monoether)(CAS Number 65545-80-4; non-HPV). US Environmental Protection Agency: Toxic Substance Control Act (TSCA) Section 8(e) Submission Received from 10/15/01 thru 12/07/01: 8EHQ-1001-14915. November 5, 2001. Available online at http://www.epa.gov/opptintr/tsca8e/doc/8esub/8e101501.htm.
[25] DuPont. 2002. DuPont flurotelomer product stewardship update, presented November 25, 2002. U.S. EPA Administrative Record AR226-1147.
[26] DuPont Haskell Laboratory. 2002. Results of a 2-week inhalation toxicity study in rats for n-diiodoperfluoro-alkanes mixture (no CAS); hexadecafluoro-1,8-diiodooctane (CAS 335-70-6); 1,1,2,2,3,3,4,4-octafluoro-1,4-diiodobutane (CAS 375-50-8); 1,6-diiodo-perfluorohexane (375-80-4); diiodofluoro chemical (?) (CAS Number 65975-18-0); non-HPV chemicals. US Environmental Protection Agency: Toxic Substance Control Act (TSCA) Section 8(e) Submission Received from 5/9/02 thru 5/22/02: 8EHQ-0502-13829D. May 7,2002. Available online at http://www.epa.gov/oppt/tsca8e/doc/8esub/2002/0509_052202.htm.
[27] Wood, LC. 2002. Thyroid Statistics. The Thyroid Foundation Of America. Available online at http://66.129.68.207/media/statistics/print (accessed 3/4/2003).
[28] US EPA (2002). Draft hazard assessment of PFOA and its salts February 20, 2002.
[29] Hill, RN., Crisp, TM., Hurley, PM., Rosenthal, SL and Singh, DV. 1998. Risk assessment of thyroid follicular cell tumors. Environ Health Perspect 106(8): 447-57.
[30] Hurley, PM. 1998. Mode of carcinogenic action of pesticides inducing thyroid follicular cell tumors in rodents. Environ Health Perspect 106(8): 437-45.
[31] Haddow, JE., Palomaki, GE., Allan, WC., Williams, JR., Knight, GJ., Gagnon, J., O'Heir, CE., Mitchell, ML., Hermos, RJ., Waisbren, SE., Faix, JD and Klein, RZ. 1999. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341(8): 549-55.
[32] Pop, VJ., Kuijpens, JL., van Baar, AL., Verkerk, G., van Son, MM., de Vijlder, JJ., Vulsma, T., Wiersinga, WM., Drexhage, HA and Vader, HL. 1999. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 50(2): 149-55.
[33] Goldenthal, EI., Jessup, DC., Geil, RG and Mehring, JS. 1978. Ninety-day subacute rhesus monkey toxicity study: Fluorad ¨ Fluorochemical FC-143. Report prepared for 3M, St. Paul, MN by Institutional Research and Devlopment Corporation (Mattawan, MN). Study No. 137-090. Reviewed in US EPA "Draft PFOA Hazard Assessment" AR226-1079.
[34] Yang, Q., Abedi-Valugerdi, M., Xie, Y., Zhao, XY., Moller, G., Nelson, BD and DePierre, JW. 2002. Potent suppression of the adaptive immune response in mice upon dietary exposure to the potent peroxisome proliferator, perfluorooctanoic acid. Int Immunopharmacol 2(2-3): 389-97.
[35] Yang, Q., Xie, Y., Alexson, SE., Nelson, BD and DePierre, JW. 2002. Involvement of the peroxisome proliferator-activated receptor alpha in the immunomodulation caused by peroxisome proliferators in mice. Biochem Pharmacol 63(10): 1893-900.
[36] Yang, Q., Xie, Y and Depierre, JW. 2000. Effects of peroxisome proliferators on the thymus and spleen of mice. Clin Exp Immunol 122(2): 219-26.
[37] Yang, Q., Xie, Y., Eriksson, AM., Nelson, BD and DePierre, JW. 2001. Further evidence for the involvement of inhibition of cell proliferation and development in thymic and splenic atrophy induced by the peroxisome proliferator perfluoroctanoic acid in mice. Biochem Pharmacol 62(8): 1133-40.
[38] George, ME and Andersen, ME. 1986. Toxic effects of nonadecafluoro-n-decanoic acid in rats. Toxicol Appl Pharmacol 85(2): 169-80. 1996. Statement from the work session on chemically-induced alterations in the developing immune system: the wildlife/human connection. Environ Health Perspect 104 Suppl 4: 807-8.
[39] Luster, MI., Dean, JH and Germolec, DR. 2003. Consensus workshop on methods to evaulate developmental immunotoxicity. Environ Health Perspect 111(4): 579-583.
[40] Jacobs, DS., DeMott, WR., Oxley, DK., Garg, U., Horvat, R., Persons, DL and Van Cott, EM, Eds. 2002. Laboratory Test Handbook. Cleveland, OH, Lexi-Comp, Inc.
[41] Gortner, EG., Lamprecht, EG and Case, MT. 1982. Oral teratology Study of T-3141CoC in rabbits. Report prepared for 3M, St. Paul, MN by Riker Laboratories. Study No. 0681TB0398.
Footnotes for informational graphic
40 ppb (1 mg/kg/day)
Decreased growth;
120 ppb (3 mg/kg/day)
Decreased growth; decreased pituitary size (females); decreased breast-feeding; [c]
370 ppb (10 mg/kg/d)
Decreased growth; decreased pituitary size (females); [c]
1,000 ppb (30 mg/kg/d)
Death; decreased growth; delayed sexual maturation (males and female); increased number of fertility cycles (female); decreased breast feeding; decreased pituitary size (females); decreased kidney size (female);
[a] Changes in organ size are often used by scientist to is a crude measure of toxicity and often reflect damaged organ function
[b] Based on adult lactating female rats 3 weeks after giving birth
[c] Effects seen in adult male rats exposed in utero. Adult male rats have higher serum levels of PFOA than adult female rats and it is unclear if the greater number of effects seen in the adult male offspring compared to the male parent never exposed in utero is due to early in life
Note: Control rats had blood PFOA levels below the level of quantitation (5.3 ppb)
PFOA pollutes air, drinking water, & food
In two pilot studies scientists found PFOA in tap water, outdoor air, green beans, apples, bread, and ground beef, from Toronto to Florida.
Table. PFOA and its precursors* have been found as contaminants in every city tested.
|
Location |
Tap Water |
Food |
Air |
Rivers, Lakes |
Wastewater Treatment Plant Discharge |
Landfill Leachate |
|
Decatur, Alabama |
not detected |
|
|
|
|
|
|
Cleveland, Tennessee |
not detected |
|
|
|
|
|
|
Mobile, Alabama |
not detected |
|
|
|
|
not detected |
|
Columbus, Georgia |
|
not detected |
|
|
|
|
|
Pensacola Florida |
not detected |
|
|
not detected |
|
not detected |
|
Port St. Lucie, Florida |
not detected |
|
|
|
|
|
|
Little Hocking, Ohio |
|
|
|
|
|
|
|
Toronto, Ontario |
|
|
|
|
|
|
|
Long Point, Ontario |
|
|
|
|
|
|
* The C10 Telomer alcohol (CF3(CF2)7CH2CH2OH) was monitored in air samples; this compound degrades to PFOA.
A blank table cell indicated that the media was not tested.
(a) Centre Analytical Laboratories, Inc. reports that the contamination in bread in Cleveland, Tennessee is suspect.
References: Multi-city environmental study: [Extract | Full Document]; Toronto air study: [Extract]; Multi-city food study: [Extract | Full Document];
Even though PFOA-based products have been sold to consumers for fifty years, neither EPA nor the manufacturers knows the relative importance of the many possible sources of PFOA in human blood. Current PFOA body burdens might come primarily from consumer products, but could also stem from exposures to PFOA contamination in the environment. Two studies conducted since 1999 shows that environmental exposures could be significant: PFOA or PFOA precursors have been found as contaminants in tap water, food, or air in every city tested.
In 1999 3M began a study to quantify the sources that lead to PFCs' "bioaccumulation in the human food chain"[1], in order to find the potential importance of environmental contamination as a source of PFOA in human blood. In six cities on the east coast, 3M tested food from supermarkets, rivers and lakes, sediment, fish, drinking water sources, tap water, influent and treated effluent from wastewater treatment plants, sludge, and municipal landfill leachate.
3M found PFCs in every city. The first test results were finalized just eight days after 3M's Scotchgard phaseout announcement (in May 2000). The company submitted data showing PFC contamination in rivers and lakes, some used as drinking water supplies. PFOA or other PFCs were found in all four states tested: Georgia, Alabama, Florida, and Tennessee. Four months later 3M reported test results showing PFCs in tap water in two of six cities tested (Columbus, Georgia and Decatur, Alabama). 3M found PFOA in the Mobile River, and in tap water in Columbus, Georgia.
As with the human blood studies, and then the wildlife studies, 3M again found PFCs where they were not expected. 3M designed the study to include three cities where PFCs chemicals were heavily used (manufacturing or supply chain cities) and three cities with no large-scale commercial use. Test results showed PFC contamination in water from all six cities. [2]
Tests of food purchased at supermarkets in six cities further confirmed widespread environmental contamination. In analyses of beef, pork, chicken, hot dogs, catfish, eggs, milk, bread, green beans, and apples purchased from grocery stores in Alabama, Tennessee, Georgia, and Florida, 3M found PFOA in five percent of the samples tested. Of the foods tested, beef, green beans, apples, and bread were all found to be contaminated with PFOA.
In air samples University of Ontario scientists found PFOA or chemicals that break down into PFOA in air - in downtown Toronto, and in rural Long Point, Ontario. 3M's test results from wastewater treatment plants and landfill leachate indicate that people are discarding or excreting significant quantities of PFOA. Scientists found PFOA in every sample in every city tested in the treated effluent from wastewater treatment plants, contamination that likely stems from human excretion or consumer products rinsed down sinks and drains - another potential source of diffuse PFOA contamination in the environment. They found PFOA in landfill leachate from two of six cities tested.
Tap Water Contamination from DuPont's Plants
"Over the years, DuPont has worked hard to reduce its chemical footprint."
—Dupont, in reference to C-8 water levels in Little Hocking, OH [5]
DuPont finds pervasive contamination around its manufacturing facilities. In internal studies of tap water in 1984 in the vicinity of their Washington Works Teflon Plant in West Virginia, DuPont detected PFOA at concentrations of 1.5 ppb in a store tap in LuBeck, West Virginia, at concentrations of 1.0 and 1.2 ppb in a store tap in Washington, WV, and at concentrations of 0.8 and 0.6 ppb in Little Hocking, West Virginia. [Extract] Many of these concentrations are higher than those detected in landfill leachate in Port St. Lucie, Florida. DuPont told neither the residents nor state regulators about the testing or the results.
When forced by the state to conduct a more thorough investigation of Little Hocking's water in 2002, the company found that levels of PFOA in the town's production wells ranged from 0.495 to 8.58 ppb. [6] These concentrations are significantly higher than DuPont's long-standing internal safety standard of 1 ppb, although DuPont has recently argued for a much higher safety standard through the auspices of a drinking water assessment team led by West Virginia regulators but which includes DuPont employees and chemistry industry consultants.
How do these chemicals get into our food,
water, and landfill leachate?
Environmental Releases. Because it is unregulated, PFOA is legally released as air and water pollution from DuPont and 3M plants in West Virginia, North Carolina, Minnesota and Alabama; and from carpet, clothing, and paper industries in North Georgia, North Carolina, and other places.
DuPont estimated (note 1) that two of its plants, its Washington Works plant in West Virginia and its Chambers Works facility in New Jersey, released a combined total of 20 tons of PFOA into the air, 3300 tons into the water and three tons into landfills in 1999. 3M estimates lower releases for its facilities: in 1997 its Cottage Grove Minneapolis production plant released 3 tons into the air, while in 1999, two PFC plants were released about 2300 tons of PFOA into the water. [7]
The immediate result of these environmental releases can be seen in the areas surrounding PFC plants. In the surface water immediately downstream of 3M's Decatur plant, concentrations of PFOA were found to be 1900 ppb and 1024 ppb. [8] In West Virginia, production wells that used to supply drinking water to DuPont's Washington Works plant have been measured to have concentrations as high as [1.9 ppb] of PFOA, and wells at landfill and digestion pond areas have reached levels as high as 13,600 ppb. [9]
Fluorotelomers Alcohols. Fluorotelomer alcohols may turn out to be the dominant source of PFOA in human blood. Through laboratory studies industry scientists have known since 1981 that fluorotelomer alcohols break down in the environment and in the body to PFOA and PFOA-like compounds. (note 2) [10, 11] [Breakdown Study | Extract | Full Document] The fluorotelomer alcohols are found in such seemingly innocuous products as hair shampoo and conditioner, paper products prepared for direct contact with food, rug cleaners, and lubricants for bicycles, garden tools and zippers.
Although large sections of the document have been removed, under claims of "Confidential Business Information," a recent DuPont Telomer Presentation in EPA's files clearly expressed DuPont's concern over the fluorotelomers:
"We are committed to and staying in the business.
We are confident that our products are safe for their intended uses."— Dupont Product Stewardship Update, 17 DEC 2001.[12] [Excerpt]
References
[1] 3M. 1999. Quality Assurance Project Plan for Empirical Human Exposure Assessment Multi-City Sampling Task. U.S. EPA Administrative Record AR226-0952.
[2] 3M. 2001. Executive Summary: Environmental monitoring - multi-city study water, sludge, sediment, POTW effluent and landfill leachate samples. U.S. EPA Administrative Record AR226-1030a111.
[3] US Environmental Protection Agency (US EPA) (2001). Analysis of PFOS, FOSA, and PFOA from various food matrices using HPLC electrospray/mass spectrometry, 3M study conducted by Centre Analytical Laboratories, Inc.
[4] Martin, JW., Muir, DC., Moody, CA., Ellis, DA., Kwan, WC., Solomon, KR and Mabury, SA. 2002. Collection of airborne fluorinated organics and analysis by gas chromatography/chemical ionization mass spectrometry. Anal Chem 74(3): 584-90.
[5] DuPont. 2002. c8 Inform. available online at http://www.c8.inform.com/dww/plant%20communications/22103.html.
[6] Association, LHW. 2002. Copies of Press Releases from Little Hocking Water Association summarizing results of C-8 levels in Ohio community water supplies (sampling rounds 1-7). U.S. EPA Administrative Record AR226-1157.
[7] US EPA (2002). Draft hazard assessment of PFOA and its salts February 20, 2002.
[8] 3M. 2001. Selected Fluorochemicals in the Decatur, Alabama Area. US Environmental Protection Agency Administrative Record Number AR226-1030a161.
[9] Taft. 2002. Compilation of Historical C-8 Data DuPont Washington Works Main Plant and Landfills. U.S. EPA Administrative Record AR226-1194.
[10] Services, PA. 2002. Biodegradation Study Report: Biodegradation Screen Study for Biodegradation Screen Study for Telomer Type Alcohols,. U.S. EPA Administrative Record AR226-1149.
[11] Hagen, DF., Belisle, J., Johnson, JD and Venkateswarlu, P. 1981. Characterization of fluorinated metabolites by a gas chromatographic-helium microwave plasma detector--the biotransformation of 1H, 1H, 2H, 2H-perfluorodecanol to perfluorooctanoate. Anal Biochem 118(2): 336-43.
[12] DuPont. 2001. DuPont Telomer Presentation. U.S. EPA Administrative Record AR226-1042.
Notes
(note 1) EPCRA Section 313 requires manufacturers to report environmental releases of only about 650 different chemicals, and PFOA is not one of them. These chemicals can be found on the TRI (Toxics Release Inventory List). http://www.scorecard.org/general/tri/tri_chem.html.
(note 2) A subsequent study sponsored by 3M reported that fluorotelomers of the general formula CF3(CF2)nCH2CH2OH will biodegrade to perfluorinated acid salts of the general formula CF3(CF2)nCO2- and CF(CF2)(n-1)CO2-.
PFCs In Animals Worldwide
After five decades of widespread use in consumer products and industrial goods and processes, PFOA has dispersed around the globe, and now contaminates wildlife on three of four continents tested. Other PFCs are even more prevalent.
In 15 studies conducted since 1994 [1-15], scientists have found PFOA in birds, fish, land and marine mammals all over the world-in 16 of 77 species, in 6 of 12 countries and on 3 of the 4 continents where animals have been tested [Wildlife study | Marine animal study]. Further studies are certain to find more contamination.
Over recent decades PFOA has evolved from a research chemical to a staple in consumer product production to a pollutant of global scope. PFOA has been found in wildlife from Italy, the US, Japan, Russia, Belgium, and Canada, and in places as remote as the Sand Island Wildlife Refuge in Midway Atoll. Scientists have detected PFOA in egg yolks of the double crested cormorant from Lake Winnipeg in Manitoba Canada; in the blood of Russian Caspian seals; and in a short-snouted spinner dolphin off the coast of Florida (Table 1).
Table 1. PFOA in wildlife.
|
Year samples were collected |
Country |
Location |
Number of animals in which PFOA was found |
Species (organ) |
Range of PFOA levels found (ppb) |
|
1991 to 2000 [6] |
US |
Gulf of Mexico |
1 of 3 |
Short-snouted spinner dolphin (liver) |
<7.5 - 20 |
|
1995 [5] |
US |
Thunder Bay, Sulphur Island, Michigan, Lake Huron |
2 of 3 |
ring-billed gull (yolk) |
<180 - 197 |
|
1991 [5] |
US |
St. Martin, Michigan, Lake Michigan |
1 of 2 |
Double-crested cormorants (whole blood) |
<29.9- 48.9 |
|
1991 to 2000 [6] |
US |
California Coast |
1 of 6 |
California Sea Lion (liver) |
<35.9 - 41 |
|
1997 to 1999 [6] |
US |
Alaska |
1 of 14 |
Polar Bear (liver) |
<8.03 - 12.9 |
|
1990 to 1998 [5] |
Midway Atoll (US Possession) |
Sand Island wildlife refuge, Midway Atoll |
1 of 3 |
Laysan albatross (liver) |
<180 - 182 |
|
1997 [5] |
Italy |
Cabras Lagoon, Sardinia |
12 of 12 |
Common cormorant (liver) |
29-444 |
|
1995 [5] |
Canada |
Lake Winnipeg, Manitoba |
2 of 4 |
Double-crested cormorants |
<180 - 245 |
|
1991 to 2000 [6] |
Russia |
Caspian Sea |
2 of 8 |
Caspian Seal (whole blood) |
<6 - 23 |
|
circa 2000 [7] |
Belgium |
Scheldtz Estuary |
8 of 26 |
Various fish species (muscle) |
< 7.5 - 46 |
|
[6] |
1991-2000 |
Baltic Sea |
1 of 81 |
Ringed Seal (liver) |
<18.8 - 39.5 |
|
1996 to 1998 [6] |
Baltic Sea |
20 of 28 |
Ringed Seal (whole blood) |
<1.25 - 9 |
|
|
1996 to 1998 [6] |
Baltic Sea |
1 of 26 |
Gray Seal (whole blood) |
<4.98 - 7 |
|
|
1991 to 2000 [6] |
Italy |
Mediterranean Sea |
2 of 4 |
Bottlenose Dolphin (whole blood) |
<2.5 - 4 |
|
1998 [12] |
Japan |
Gyotoku bird observatory, Chiba, Tokyo |
1 |
Black-headed gull (liver) |
21 |
|
1999 [12] |
Japan |
Atsugi, Kanagawa |
1 |
Black-eared kite (liver) |
21 |
|
[5] |
Japan |
Not reported |
2 of 7 |
Sea Eagle |
<3.35 - 6.2 |
PFCs as global contaminants. Although PFOA widely contaminates wildlife, other PFCs are even more prevalent. Because PFOA and other PFCs appear to be toxic by common mechanisms, and to target common organs and systems in animals, the combined influence of multiple PFCs drives health risks to wildlife.
Like DDT, PCBs and dieldrin in the 1970s, PFOS, PFOA and other perfluorinated and polyfluorinated compounds have been distributed across the globe. They have been found in animals living in the most pristine environments in the most remote locations. Despite limited testing for the presence of PFCs in the environment, they have been detected in 76 of 98 species tested, in 14 countries and 3 out of the 4 continents on which specimens were collected. PFOS, used by 3M in Scotchgard products until 2000, is the most widely detected:
"PFOS was detected in all of the wildlife species analyzed." -Giesy and coworkers on the results of their study of 175 liver and blood samples of Marine Mammals, Fish, and Birds from the Baltic Coasts and the Mediterranean Sea.[10]
3M's evidence of global contamination in the 1980s. Through studies conducted in the late 1980s, 3M learned that terminal breakdown products of many PFCs - PFOA and PFOS, for example - will not break down in the environment. These laboratory studies covered all the basic mechanisms by which chemicals are known to break down - in sunlight or through reactive chemicals in the air (photolysis), by bacterial action (biodegradation), and through chemical reaction with water (hydrolysis). 3M found that terminal PFCs are completely resistant to all of these processes.[16-20]
Through other studies that define parameters like vapor pressure and Henry's constant, 3M learned that some PFCs have the potential to travel long distances through the atmosphere. These studies, combined with the degradation data, pointed to the likelihood that PFCs have been migrating for decades to remote areas far from manufacturing facilities and urban centers.
Even though all the basic data were in place to indicate a global problem, not until the late 1990s did 3M test the theory of global contamination. In 1997 their laboratory identified PFOS contamination in blood bank supplies that were being used as control samples against contaminated worker blood. Further blood studies that pointed to the possibility of widespread contamination of human blood. During these studies 3M staff acquired and tested 60 bird livers from the National Wildlife Health Center in Madison, Wisconsin as a first step in defining the scope of wildlife contamination.
3M's definitive wildlife studies in the late 1990s. 3M's lab found PFCs in more than three-quarters of 60 bird livers samples collected from across the continental US [5]. The lab reported PFCs in every liver sample from white pelican, double crested cormorant, and great blue heron and in all but one brown pelican sample - a total of 44 detections in 45 bird liver samples from California, Louisiana, Florida, and Nevada. The chemicals the scientists detected included the Scotchgard chemical PFOS, a PFOS precursor called PFOSA, and PFHS, the 6-carbon sister chemical of PFOS.

In contrast, the lab found PFCs in 20 percent (3) of 15 sandhill crane livers analyzed, in samples from Nebraska, Arizona, and New Mexico, and in just one of those samples were PFCs at levels high enough to quantify: the liver of a Nebraska crane [5]. The different PFC profiles among bird species correlate with diet. The study showed that birds subsisting on fish are more likely to be contaminated with PFCs, indicating that, like PCBs and DDT, these chemicals concentrate in fish with the potential to build up in the food chain.
Over a four-day period beginning March 15 1999, 3M's environmental laboratory conducted additional tests of wildlife tissue designed to find the outer bounds of PFC contamination globally. 3M scientists tested for PFOS and related PFCs in eaglet blood collected from remote areas of the U.S. If this blood were found to be free of PFCs, scientists could work backwards to determine how far the contamination had spread from urban areas where Scotchgard and other PFC products are used.
3M found PFCs in all five eaglets tested [14]. Nestling eaglets who had never flown were contaminated with PFOS at levels ranging from 30 to 77 parts per billion in the blood. One likely source of exposure was the fish from their mothers - from the waters around Devil's Lake, Slate River Falls, Pickerel Channel, and Caulkins Creek in the Michigan's Upper and Lower Peninsulas, and from Steve's Island in Minnesota's remote Voyageurs National Park. Subsequent tests have shown that even the yolks of wild bird eggs are contaminated with PFCs [5]. These tests, combined with 3M's concurrent human blood testing, gave final confirmation that PFCs contaminate people and wildlife on a global level.
In early 2000 scientists from Michigan State University completed testing 247 liver, kidney, and blood samples from 15 species of marine mammals - including polar bears, sea lions, seals, and whales collected from the Arctic, Sable Island in Canada, Alaskan coastal waters, Florida, and California. PFOA was found in 17 percent of the samples tested, but the findings for PFOS pointed to a nearly complete pervasiveness of PFC contamination. The authors of the new marine mammal study concluded: "The occurrence of PFOS in marine mammals from the Arctic waters suggests widespread global distribution of PFOS including remote locations."[9].
All the wildlife studies conducted to date indicate that of the PFCs tested, 3M's Scotchgard chemical PFOS has achieved the greatest distribution and the highest concentrations in wildlife. Scientists have detected PFOS in wildlife at concentrations above those found in plant workers. In four Caspian tern eggs in Michigan lab analyses showed an average whole egg concentration of 2605 ppb[5], and tests of nine minks in South Carolina showed an average liver concentration of 2085 ppb[4]. These levels are higher than whole blood concentrations of five cell operators in 3M's PFC manufacturing plant in Decatur, Alabama, who averaged 1970 ppb[21].
PFOS has been detected in the most protected species and in the most pristine environments on earth. It has been detected in Alaskan polar bears (in 17 of 17 tested polar bears, at an average liver concentration of 350 ppb)[10], Midwestern bald eagle nestlings younger than 70 days old (in 33 nestlings all under 70 days old, with an average blood plasma concentration of 330 ppb)[5], a great egret from Swan Lake National Wildlife Refuge in Sumner, Missouri (liver concentration of 171 ppb)[5], bottlenose dolphins from the Adriatic Sea off the coast of Riccione, Italy (detected in 4 of 4 bottlenose dolphins, with an average blood concentration of 143 ppb)[7], and Laysan albatrosses from Sand Island, a wild life refuge in Midway Atoll (detected in 6 of 6 albatrosses, average blood concentration of 16 ppb)[5].
PFOSA, a chemical precursor of PFOS, is detected at significant levels in species across the globe. Off of the Italian coast, PFOSA (also known as FOSA) has been detected in a Delphinus Whale (liver concentration was 878 ppb)[7] , and in Swordfish (detected in 7 of 7 swordfish, average blood concentration was 7 ppb)[7].
References:
[1] Giesy, JP and Kannan, K. 2001. Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol 35(7): 1339-42.
[2] Giesy, JP and Kannan, K. 2001. 3M Company submitted report: Accumulation of perfluorooctanesulfonate and related fluorochemicals in fish tissues. U.S. EPA Administrative Record AR226-1030a.
[3] Giesy, JP and Kannan, K. 2002. Perfluorochemical surfactants in the environment. Environ Sci Technol 36(7): 146A-152A.
[4] Giesy JP, KK. 2001. Accumulation of Perfluorooctanesulfonate and related Fluorochemicals in Mink and River Otters. U.S. EPA Administrative Record AR226-1030a157.
[5] Giesy JP, KK. 2001. Perfluorooctanesulfonate and Related Fluorochemicals in Fish-Eating Water Birds, AR226-1030a159. U.S. EPA Administrative Record AR226-1030a159.
[6] Giesy JP, KK. 2001. Accumulation of Perfluorooctanesulfonate and related Fluorochemicals in Marine Mammals. U.S. EPA Administrative Record AR226-1030a160.
[7] Giesy JP, KK. 2001. Accumulation of Perfluorooctanesulfonate and Related Fluorochemicals in Fish Tissues. US Environmental Protection Agency Administrative Record Number AR226-1030a156.
[8] Kannan, K., Franson, JC., Bowerman, WW., Hansen, KJ., Jones, PD and Giesy, JP. 2001. Perfluorooctane sulfonate in fish-eating water birds including bald eagles and albatrosses. Environ Sci Technol 35(15): 3065-70.
[9] Kannan, K., Koistinen, J., Beckmen, K., Evans, T., Gorzelany, JF., Hansen, KJ., Jones, PD., Helle, E., Nyman, M and Giesy, JP. 2001. Accumulation of perfluorooctane sulfonate in marine mammals. Environ Sci Technol 35(8): 1593-8.
[10] Kannan, K., Corsolini, S., Falandysz, J., Oehme, G., Focardi, S and Giesy, JP. 2002. Perfluorooctanesulfonate and related fluorinated hydrocarbons in marine mammals, fishes, and birds from coasts of the Baltic and the Mediterranean Seas. Environ Sci Technol 36(15): 3210-6.
[11] Kannan, K., Newsted, J., Halbrook, RS and Giesy, JP. 2002. Perfluorooctanesulfonate and related fluorinated hydrocarbons in mink and river otters from the United States. Environ Sci Technol 36(12): 2566-71.
[12] Kannan, K., Choi, JW., Iseki, N., Senthilkumar, K., Kim, DH and Giesy, JP. 2002. Concentrations of perfluorinated acids in livers of birds from Japan and Korea. Chemosphere 49(3): 225-31.
[13] Kannan, K., Hansen, KJ., Wade, TL and Giesy, JP. 2002. Perfluorooctane sulfonate in oysters, Crassostrea virginica, from the Gulf of Mexico and the Chesapeake Bay, USA. Arch Environ Contam Toxicol 42(3): 313-8.
[14] 3M. 1998. Screening of PFOS levels in eagles and albatross. U.S. EPA Administrative Record AR226-0080.
[15] 3M Laboratory report of fluorochemicals in wild bird livers, Report prepared for 3M, St. Paul, MN by 3M Environmental Laboratory Fluorine Analytical Chemistry Team (FACT). Study No. FACT-TOX-010.
[16] 3M. 2001. Screening Studies in the Aqueous Photolytic Degradation of Perfluorooctanoic Acid (PFOA). U.S. EPA Administrative Record AR226-1030 Photolysis E00-2192.
[17] 3M. 2001. Hydrolysis Reactions of Perfluorooctanoic Acid (PFOA). U.S. EPA Administrative Record AR226-1030a090.
[18] 3M. 2000. Biodegradation study of PFOS. US Environmental Protection Agency Administrative Record Number AR226-0057.
[19] Company, M. 1976. Biodegradation Studies of Fluorocarbons. U.S. EPA Administrative Record AR226-0356.
[20] 3M. 1978. The 18-Day Aerobic Biodegradation Study of Perfluorooctanesulfonyl-Based Chemistries. U.S. EPA Administrative Record AR226-1030a.
[21] Olsen GW, LP, Simpson CA, Burris JM, Burlew MM, Lundberg JK, Mandel JH. 2001. Descriptive Summary of Serum Fluorochemical Levels among Employee Participants of the Year 2000 Decatur Fluorochemical Medical Surveillance Program. Final Report Epidemiology, 220-3W-05. U.S. EPA Administrative Record AR226-1030a120a.
DuPont’s Spin About PFOA
April 2003
DuPont is in big regulatory trouble at EPA. And that could mean big economic trouble for the company. In response to increasing, unfavorable press reports DuPont has repeatedly made claims that exposures to PFOA, or C8, do not present a human health risk. This public relations spin is clearly out of step with recent conclusions drawn by the US EPA, with data published in peer-reviewed journals, and with data embedded in 50,000 pages of industry-sponsored studies submitted to EPA, which EWG has reviewed and posted in searchable form online (click for searchable database).
In summary, here is DuPont's spin on PFOA's health risks and EWG's analysis of current scientific knowledge:
- DuPont asserts that levels of PFOA found in human blood are too low to be a health risk. This conclusion is contradicted by recent findings from an industry-sponsored rat reproduction study. After seeing this study, the EPA initiated a “priority review” of PFOA to determine whether expedited regulatory action against the compound is warranted under Section 4(f) of the Toxic Substances Control Act (TSCA) due to concerns that the compound causes adverse developmental and reproductive effects in animals. The most recent step in EPA’s priority review is a draft risk assessment that shows blood levels of PFOA in women and girls are dangerously close to blood levels of PFOA that cause serious harm to laboratory animals.
- DuPont claims there is no evidence or data that demonstrates PFOA causes adverse human health effects. DuPont has reported only one health study in the public record on workers exposed to PFOA, and this study was limited to measuring liver enzyme levels, which were significantly increased in workers. More troublesome are findings from studies sponsored by 3M showing that workers with high PFOA exposures are significantly more likely to die of prostate cancer and cerebrovascular disease. Another PFOA study sponsored by 3M found that exposed workers are significantly more likely to seek care for prostate cancer, gastrointestinal tract lesions, biliary tract and pancreatic disorders, and urinary bladder inflammation. Interpreting these studies is complicated because they all have significant design flaws and are based on small numbers of people. Still, cancer and other disease relationships are found, and the consistency of health effects among workers and in laboratory studies is striking.
- DuPont denies that PFOA causes reproductive or developmental toxicity. EPA’s priority review of PFOA was initiated precisely due to concerns raised by EPA scientists regarding the developmental and reproductive toxicity of PFOA in animal studies.
- DuPont emphasizes that the liver is the most important target organ for PFOA toxicity. PFOA causes toxicity to virtually every organ or system tested, including the brain, pituitary, adrenal gland, thyroid, ovary, male reproductive tract, immune system and kidney. PFOA also causes mammary, testicular, pancreatic and liver tumors. Effects on the ovary, pituitary, kidney, spleen and seminal vesicles were affected by PFOA at or below doses where liver effects were observed.
- DuPont claims that PFOA causes toxic effects in laboratory animals by a single “mechanism of action” that is not relevant to people. PFOA causes adverse effects by at least five mechanisms of action, four of which are clearly relevant to humans. For only one of these mechanisms, “peroxisome proliferation,” are scientists debating the relevance to humans. Peroxisome proliferation is clearly linked to liver toxicity in rats. Scientists do not know if peroxisome proliferation is responsible for the liver toxicity observed in monkeys or high liver enzyme levels observed in workers, or whether a different mechanisms causes these effects. PFDA (C10), a sister chemical to PFOA, has been shown to cause peroxisome proliferation in human brain cells.
DuPont Spin vs. EWG Analysis
Relationship between human blood levels of PFOA and adverse effects in laboratory animals
|
Dupont spin. On March 18, 2003, DuPont staff presented the following information to the media, implying that humans would have to drink more than 2000 gallons of contaminated drinking water to reach exposures that harm laboratory animals [1] [slide 15]: Assessing the Science: Is the dose (in animal studies) relevant?
Reproduction of DuPont graphic |
EWG Analysis. DuPont has both misrepresented the “no effect” levels of these studies and implied that people could never be exposed to PFOA at levels high enough to approach the dose levels given to laboratory animals. In none of the studies DuPont refers to did scientists find a dose that did not cause significant harm to laboratory animals [EWG critical study summary table | EPA Hazard Assessment - PDF pages 60, 77, 78-79].
More disturbing is EPA’s conclusion that levels of PFOA in the general population are approaching the serum levels found in female rats fed 10 mg/kg/day, or the equivalent of drinking “>40,000 gal. per day” according to DuPont. Because people clearly do not drink anything approaching 40,000 gallons of water a day, the only interpretation of EPA’s analysis is that DuPont can’t explain how people all over the United States could have accumulated such substantial amounts of PFOA.
Residents living near DuPont Washington Works facility near Parkersburg, West Virginia who are exposed to PFOA through local contamination in air and drinking water, likely have higher PFOA blood levels than the levels in the general population about which EPA has expressed concern. DuPont has estimated that people living near Washington Works may have blood levels that exceed 1 part per million (ppm) by drinking water contaminated with 3 parts per billion (ppb) of PFOA for six years [Extract and EWG analysis].
This level of contamination in drinking water (3 ppb) is 50 times lower than a controversial drinking water screening level recently set by the West Virginia Department of Environmental Protection [2,3] [View news release]. Female rats with blood levels this high gave birth to pups that died early in life and had delayed sexual maturation, decreased growth, altered reproductive cycling, and showed damage to the liver, pituitary, brain, seminal vesicles, testes, epididymis, spleen and thymus in adulthood[4].
Table 1. Effects noted in critical PFOA studies (see more about effects noted in critical studies)
|
Effect (Animal Model) |
No Observed Effect Level |
Effects observed at the lowest dose tested |
|
Chronic-liver |
DuPont claim: 0.5 mg/kg Fact: DuPont did not even test 0.5 mg/kg in the chronic primate study [Extract] Fact: PFOA caused effects at every dose [Full document PDF page 60] |
3 mg/kg/day: one of four monkeys died, (the monkey had had hind-limb paralysis, muscle incoordination and did not respond to touch); increased liver weight; and increased total bilirubin, a pigment in bile that comes from hemoglobin in old red blood cells |
|
Cancer |
DuPont claim: 2 mg/kg (~ 1.6 mg/kg/d or 30 ppm) Fact: PFOA caused effects at every dose, including 1.6 mg/kg/d (30 ppm) [Full document PDF page 79] |
1.6 mg/kg/day: cellular changes in the ovary, lung (female); salivary gland (male); muscle incoordination (female) and possible thyroid tumor (male) |
|
Reproduction/ Developmental |
DuPont claim: 10 mg/kg Fact: PFOA caused effects at every dose in male rats (1, 3, 10 and 30 mg/kg/d) [Full document PDF page 77] |
1 mg/kg/d (male effects): decreased growth; damage to liver, kidney, seminal vesicle and spleen |
Source: Compiled by Environmental Working Group.
PFOA and worker effects
|
DuPont spin. In the same March 18, 2003 media presentation, on slide 18, DuPont staff incorrectly state that no health effects have been observed in workers[1]: Assessing the Science
Reproduction of DuPont graphic |
EWG Response:
PFOA linked to changes in liver enzymes. Belying the certainty of these claims, DuPont has reported only one health study in the public record on workers exposed to PFOA, and this study was limited to measuring liver enzyme levels, which were significantly increased in workers [Extract | Full document]. This study was conducted in 1981[5] in response to concerns raised in a memo from DuPont medical staff that chemical plant workers at the Washington Works plant were more likely to have high liver enzyme levels, indicating abnormal liver function. Marked “personal and confidential” DuPont medical staff noted that “My preliminary results suggest that C-8 [PFOA] exposed workers may possibly have positive liver function tests more often than the plant population as a whole, and that the number of active wage roll employees having myocardial infarction from 1974 through 1977 was somewhat higher than expected based on Company-wide experience”[6] [Extract]. DuPont failed to track cardiovascular disease in the follow-up study. Abnormal liver function tests are routinely found in laboratory animals exposed to PFOA.
PFOA linked to changes in mortality patterns among workers. Mortality studies have been conducted at 3M plants in Cottage Grove, MN[7,8][EWG Worker Study Document]. In these studies, serum levels of fluorochemicals were not measured. Instead, exposure was estimated based on job function. Cause of death was determined via death certificates. Long-term workers in the Chemical Division at 3M’s Cottage Grove, MN plant were found to be 3.3 times more likely to die from prostate cancer when compared to the workers who did not work in the Chemical Division[8] [Extract].
A follow-up mortality study found that workers with definite PFOA exposure were up to 15 times more likely die from cerebrovascular disease[7][Extract]. Other causes of death that were increased in Chemical Division workers, although not statistically significant, were cancers of the testis and pancreas [EWG Worker Study Document]. These studies have involved so few people, however, that a single incidence of cancer can change study results from a finding of “no increase” to “statistically significant increase.” However, it is notable that PFOA damages the prostate[4] and causes both testicular and pancreatic tumors in rats[9,10]. In addition, 3M workers at the Cottage Grove, MN plant were more likely to die from bladder cancer, regardless of job function, than the general population[11].
3M also conducted an “episode of care” study. It was conducted at the Decatur, AL plant to assess why DuPont workers sought medical care. Workers with the highest and longest exposure to perfluorochemicals were significantly more likely to seek care for prostate cancer, gastrointestinal tract neoplasms (especially benign colonic polyps), and disorders of the biliary tract and pancreas, cystitis and lower urinary tract infections[12][EWG Worker Study Document]
PFOA linked to cancer in animal and worker studies. Four of five tumor types caused by PFOA or PFOS in animals (liver, testicular, breast, and thyroid) have been significantly increasing in Americans during the last 10 to 25 years[13-15][Extract]. PFOA causes three of these tumor types - liver, testes and breast - in rats[16 pp. 78-79]. PFOA has been associated with statistically significant increases in prostate cancer death and with 3M workers seeking medical care for prostate cancer (in PFOA plant workers in two separate studies[7,12]). Other short-term studies show that chemicals that break down into PFOA cause cellular changes in the thyroid consistent with a “mechanism of action” known to lead to thyroid tumors in rodents [17-23].
PFOA linked to changes in cholesterol, reproductive hormones, and growth hormones in worker studies, animal studies, or both. Increased serum PFOA levels are significantly associated with increased triglyceride and cholesterol levels and decreased high-density lipoprotein (HDL) or “good” cholesterol. PFOA was also positively associated with triiodothyronine (T3), one type of thyroid hormone[24,25].
Analysis of hormone levels in blood collected from 3M's Cottage Grove workers in 1993 and 1995 found that workers in the highest exposure PFOA category (> 30 ppm in blood) had 10 percent higher estradiol levels than other PFOA employee groups. This effect was not statistically significant, which could be due to the small sample size in the high exposure group (only 4 in 1993 and 5 in 1995)[26]. An earlier study also found a relationship between PFOA and estrogen [29] [Extract | Full document]. PFOA has been shown to increase estradiol in animal studies[27]. Increased levels of estradiol cause significant reproductive damage in males[28]. Workers in the high exposure category also had increased levels of thyroid stimulating hormone, a signal that workers may be at risk for hypothyroidism[26,29] [Extract]. Hypothyroidism affects an estimated 4.6 percent of people in the US, mostly women, and is linked to fetal brain damage[30,31]. Because so few 3M chemical plant workers are women, they have limited ability to study deseases and reproductive function in women.
PFOA and cancer
|
DuPont spin. On slide 11 of the March 18 2003 media presentation, DuPont staff misleadingly state that PFOA is not a human carcinogen[1]: Analysis of PFOA Health Effects
Reproduction of DuPont graphic |
EWG Response: DuPont unequivocally told the media on March 18, 2003 that PFOA is not a human carcinogen, yet DuPont has never reported cancer incidence in their workers exposed to PFOA. In fact, the last public record of DuPont studying the health of workers with high PFOA exposures was in 1981, and in this study DuPont looked only at liver enzyme levels, not cancer or any other health outcomes.
3M, the original producer of PFOA, has also never studied cancer incidence in workers exposed to the chemical, but they did study cancer mortality. They found that chemical division workers were more likely to die from prostate cancer than less exposed workers[8][Extract | Full Document]. Workers are also more likely to die of cerebrovascular disease [Extract]
3M also conducted what is referred to as “an episode of care study,” in which they found that PFOA-exposed workers were more likely to seek treatment for cancers of the male reproductive tract (including prostate cancer) compared to workers 3M believes are exposed to lower amounts of PFOA[12] [EWG Worker Study Document].
According to the latest EPA proposed cancer guidelines, released earlier this year, PFOA would probably be classified as a likely human carcinogen because it meets several proposed EPA criteria for this category[16 p. 62]:
• “An agent that has tested positive in more than one species, sex, strain, site, or exposure route, with or without evidence of carcinogenicity in humans;”
PFOA causes tumors in both male rats (testicular, liver, and pancreatic tumors) and female rats (mammary tumors), thus meeting this criterion by testing positive in more than one sex and in more than one site.
• “A positive study that is strengthened by other lines of evidence, for example, some evidence of an association between human exposure and cancer (but not enough to infer a causal association), or evidence that the agent or an important metabolite causes events generally known to be associated with tumor formation (such as DNA reactivity or cell growth control) likely to be related to the tumor response in this case; “
The cancer findings for PFOA to date are not sufficient to draw conclusions of causality in humans, but there is a striking concordance between evidence of cancer in PFOA workers and known PFOA target organs in animals [EWG Worker Study Document]. PFOA meets this criterion because it inhibits the ability of liver cells to communicate with each other, called gap junction intercellular communication (GJIC)[32].
Normally, cells do not multiply unchecked because neighboring cells secrete chemical signals, by processes like GJIC, to prevent excessive cell multiplication. Therefore, when GJIC is decreased, cells – including cancer cells- can grow unchecked. Decreased GJIC is a sign that PFCs could be acting as tumor promoters, which means that even if they do not directly damage DNA, they make it easier for a cancer cell to multiply and produce more cancer cells, eventually resulting in a tumor.
Finally, PFOA increases production of estradiol, a potent form of estrogen, by increasing liver activity of the enzyme that converts testosterone to estradiol (aromatase)[27,33]. Male workers at one of 3M's plants may also have increased blood levels of estrogen [26,29]. Although estrogen is required for normal body function, too much estrogen is a risk factor for certain types of cancer. For example, the type of estrogen used in hormone replacement therapy (HRT) is classified as a known human carcinogen, linked to uterine and breast cancer[34]. Increased estrogen levels have been implicated in testicular cancer[35] and abnormal prostate growth[28,36].
Four of five tumor types caused by PFOA or PFOS in animals (liver, testicular, breast, and thyroid) have been significantly increasing in Americans during the last 10 to 25 years[13-15][Extract]. PFOA causes three of these tumor types - liver, testes and breast - in rats[16 pp. 78-79]. Other short-term studies show that chemicals that break down into PFOA cause cellular changes in the thyroid consistent with a “mode of action” known to lead to thyroid tumors in rodents [17-23].
PFOA and developmental/reproductive toxicity
|
DuPont spin. Also, in Slide #11 from DuPont’s public presentation on March 18, 2003, DuPont staff state that PFOA is not a reproductive or developmental toxin, even after EPA initiated a priority review because of their concerns over results from a 2002 reproduction study: Analysis of PFOA Health Effects
Reproduction of DuPont graphic |
EWG response: Far from considering PFOA “not developmentally toxic,” the U.S. EPA has so far concluded just the opposite. EPA developed a risk assessment as part of a “priority review” of PFOA to determine whether expedited regulatory action against the compound is warranted under Section 4(f) of the Toxic Substances Control Act (TSCA). EPA is considering this action because (according to the agency) “concerns for developmental toxicity were raised from the results of a rat two-generation reproductive toxicity study of APFO [PFOA]”[35 p. 56].
Based on powerful results from rat reproduction studies, and a comparison of blood levels in the affected animals with blood levels in people, EPA scientists have so far concluded that children with the highest measured blood levels of PFOA have less than one tenth the protection, or less than one tenth the margin of safety, than the level the agency considers to be safe. In EPA parlance, the “margin of exposure” for the most exposed children is just 7, when it should normally be at least 100[35 p. 51].
According to EPA scientists, PFOA causes “significant increases in treatment related deaths”[37 - PDF p. 37] [Full document] in offspring at doses that did not affect the mothers, and a range of serious changes in the weight of various organs, including the brain (decrease), prostate (decrease), liver (increase), thymus (decrease), pituitary (decrease) and kidneys (increase in lower dose groups, decrease in high dose group)[80 PDF pg 179-191] [Full document]. Both male and female offspring had delayed sexual maturation[4,37 PDF page 38-39] [Full document]. The deaths of a significant number of rat pups within 2 to 4 days after weaning in experiments in which the mother was exposed to PFOA is highly unusual, and raises grave concerns about the toxicity of PFOA to people.
In addition, PFOA causes a significant increase in low birth weight pups in animal studies. Low birth weight is recognized as a risk factor for insulin resistance or Type II diabetes, high blood pressure, and cardiovascular disease later in life[38,39]. Studies have also shown that health risks remain even for those low–birth weight children who achieve normal weight later in childhood, including risks for high blood pressure, stroke, insulin resistance and glucose intolerance[40-46].
Other studies have shown that PFOA causes numerous effects on male and female reproductive organs, such as testicular tumors[9,10,27], decreased sperm production[9], mammary gland tumors[9] and cellular effects in the ovary[9]. PFOA also affects the prostate gland, seminal vesicle, epididymis and testis[4], and increases estrogen levels[27,47,48].
PFOA and liver toxicity
|
Dupont spin. From the March 18, 2003 media presentation on slide #13, DuPont staff state that rats are the most sensitive animals model and that liver toxicity is the most sensitive effect: Is the effect relevant?
Reproduction of DuPont graphic |
EWG Response: The rat is the most studied animal model for PFOA, but may not be the most sensitive. Only two studies have looked at the effect of PFOA in monkeys[49,50], the laboratory species that is closest to humans biologically. It is difficult to say whether the rat or monkey is more sensitive because industry scientists have not been able to identify a dose of PFOA that does not cause harm to both species. One of four monkeys died in the lowest dose group in a 6-month PFOA exposure study [49] [Extract]. In addition, neither 3M nor DuPont has conducted a chronic exposure or reproduction study in monkeys; the longest study conducted to date equates to a mere 3.3% of a monkey’s lifespan and is equal to a little over two years exposure in people[51]. Moreover, the longest exposure monkey study for PFOA did not include females; only male monkeys were tested[49]. In their latest assessment of risk, EPA concludes that females are more sensitive than males to the effects of PFOA.
The liver is the most studied organ for PFOA, but is not the most sensitive. PFOA causes toxicity to virtually every organ or system tested, including the brain, pituitary, adrenal gland, thyroid, ovary, male reproductive tract, immune system and kidney. PFOA also causes mammary, testicular, pancreatic and liver tumors[35].
Both the rat cancer and reproduction study suggest that liver enlargement is just one of several sensitive effects. Effects on the ovary, pituitary, kidney, spleen and seminal vesicles were affected by PFOA at or below doses where liver effects were observed. In female rats, adverse effects on the ovary[9] occur at a dose 10 times lower than the dose that caused liver toxicity[Extract | Full document]. Likewise, in the rat reproduction study, significant increases in the number of dead pups and decreased pituitary size occurred in females exposed to PFOA at doses that did not cause liver enlargement. The male offspring in the reproduction study showed changes in four different organ weights at the lowest dose – the kidney, spleen, liver and seminal vesicle[4].
In monkeys, PFOA caused liver enlargement at the same dose that resulted in death for one of four monkeys tested[49][Extract]. DuPont has no idea what killed the monkey, so they cannot rule out that non-liver toxicity resulted in the monkey death[35 p. 54; p. 58].
Industry research does not establish what is causing the liver enlargement found in monkeys, which are more closely related to humans. Industry scientists found that PFOA increased the size of the liver in monkeys at every dose they studied, and cellular studies point to mitochondrial damage, not peroxisome proliferation[35 p. 5, p. 9] as the mechanism of action. Moreover, 3M and DuPont workers exposed to PFOA often have altered liver enzyme levels that signal liver damage[5,6,25,52]. Simply put, the worker findings and monkey studies show that PFOA liver toxicity is relevant to humans.
Relevance of laboratory animals findings to humans
|
DuPont spin. Also on slide #13, DuPont staff imply that the effects observed in laboratory animals are not relevant to humans: Is the effect relevant?
Reproduction of DuPont graphic |
EWG Response: PFOA causes adverse effects by at least five mechanisms of action, four of which are clearly relevant to humans. The five mechanisms of action that have been identified for PFCs so far are: mitochondrial toxicity; cell membrane disruption that results in decreased cell communication; peroxisome proliferation; increased production of estrogen; and decreased thyroid hormone levels or hypothyroidism.
The relevance of only one of these mechanisms of action to people, called “peroxisome proliferation,” is currently under debate. Peroxisome proliferation is clearly linked to liver toxicity in rats, but it is unknown whether peroxisome proliferation or another mechanism of action is responsible for the liver toxicity observed in monkeys or high liver enzyme levels observed in workers. Peroxisome proliferation cannot account for all of the toxic effects of PFOA. PFOA causes toxicity to virtually every organ or system tested, including the brain, pituitary, adrenal gland, thyroid, ovary, male reproductive tract, immune system and kidney. PFOA also causes mammary, testicular, pancreatic and liver tumors. Effects on the ovary, pituitary, kidney, spleen and seminal vesicles were affected by PFOA at or below doses where liver effects were observed.
The relevance of peroxisome proliferation to humans is a subject of current debate. A decision in 1995 by the International Agency for Research on Cancer (IARC) held that peroxisome proliferation is not relevant to people as a mode of action for causing cancer but this conclusion has come under scrutiny[53-55]. In recent critiques, a leading government scientist notes that several human medications are peroxisome proliferators, so clearly humans respond to these chemicals. In addition, the IARC panel inexplicably ignored a critical paper that found increased peroxisome enzyme activity in human liver cells following exposure to another chemical peroxisome proliferator[54,56]. Recently, IARC panels have come under heavy criticism from numerous scientists as being too heavily weighted with industry scientists [53,54,57-62].
Arguments over the relevance of peroxisome proliferation to humans may be moot for PFOA because a sister chemical, C10 or PFDA, has been shown to cause peroxisome proliferation in human brain cells (liver cells were not studied)[63] [Extract].
Industry-sponsored studies disproportionately test effects of peroxisome proliferation over effects driven by other mechanisms of action. For PFOA, other major toxic effects are altered lipid metabolism, pancreatic tumors and damage to the immune system, thyroid, and reproductive tract (including testicular and mammary gland tumors)[4,10,27,33,49,64-70].
This “disproportionate testing of toxic effects” is extremely clear for C10, a sister chemical to PFOA that differs only by the addition of 2 carbon and 4 fluorine atoms. Between, 1985 and 1992, nine studies were published describing the effects of C10 (PFDA) on lipid metabolism and the thyroid gland, immune system and reproductive tract[71-79]. No studies published since 1992 has followed up one the dramatic effects in the thyroid, immune system and reproductive tract described between 1985 and 1992. C10 is also found in human blood [Extract | Full document], but has not yet come under regulatory scrutiny from EPA although its toxicity profile is virtually identical to PFOA, differing only in potency.
References
[1] DuPont (2003). March 18, 2003 DuPont media briefing presentation by Paul Bossert, plant manager of DuPont Washington Works.
[2] West Virginia Department of Environmental Protection (WVDEP) (2002). Press Release: Health Level for C8 announced by expert team.
[3] Hilderliter, PM and Jepson, GW. 2001. A simple, conservative compartmental model to relate ammonium perfluorooctanoate (APFO) exposure estimates of perfluorooctanoate (PFO) blood levels in humans. DuPont Haskell Laboratory for Health and Enviornmental Sciences.
[4] York, RG (2002). Oral (gavage) two-generation (one litter per generation) reproduction study of ammonium perfluorooctanoate (APFO) in rats. Report prepared for 3M, St. Paul, MN by Argus Research (Horsham, PA). Sponsor's Study No. T-6889.6., US EPA Adminstrative Record AR226-1092, Reviewed in US EPA Adminstrative Record AR226-1137 (pages 282-295; PDF pages 179-191).
[5] Fayerweather, WE. 1981. Liver study of Washington Works employees exposed to C-8: results of blood chemistry testing (not published).
[6] DuPont. 1979. Personal and confidential memo from Dr. Fayerweather (epidemiologis) to Dr. Power (medical superintendent): Status report on Washington Works liver function survey and coronary heart disease mortality study - August 28. 1979.
[7] Alexander, B (2001). Mortality study of workers employed at the 3M Cottage Grove facility. Final Report. Division of Environmental and Occupational Health, School of Public Health, University of Minnesota, April 26, 2001, Reviewed in U.S. EPA Administrative Record AR226-1137 (pages 143-146; PDF pages 40-43).
[8] Gilliland, FD and Mandel, JS. 1993. Mortality among employees of a perfluorooctanoic acid production plant. J Occup Med 35(9): 950-4. Also reviewed in EPA Administrative Record document AR226-1137 (pages 140-42; PDF pages 37-39).
[9] Sibinski, LJ. 1987. Two-Year oral (diet) toxicity/carcinogenicity study of fluorochemical FC-143 (perfluorooctane ammonium carboxylate) in rats. Report prepared for 3M, St. Paul, Minnesota by Riker Laboratories Inc. Study No. 0281CR0012; 8EHQ-1087-0394, October 16, 1987 Reviewed in US EPA "Revised Draft PFOA Hazard Assessment-Robust Study Annex" AR226-1137, (pp. 260-267; PDF pp 157-164).
[10] Biegel, LB., Hurtt, ME., Frame, SR., O'Connor, JC and Cook, JC. 2001. Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats. Toxicol Sci 60(1): 44-55.
[11] Alexander, B (2001b). Mortality study of workers employed at the 3M Decatur facility. Final Report. Division of Environmental and Occupational Health, School of Public Health, University of Minnesota, April 26, 2001, Reviewed in “Letter from Mr. Charles Auer and Draft Hazard Assessment of PFOA and its Salts with Page 8 was corrected on 4/15/02” U.S. EPA Administrative Record AR226-1093.
[12] Olsen, GW., Burlew, MM., Hocking, BB., Skratt, JC., Burris, JM and Mandel, JH. 2001. An epidemiologic analysis of episodes of care of 3M Decatur chemical and film plant employees, 1993-1998. Reviewed in US Environmental Protection Agency Administrative Record AR226-1137 (pages 156-159; PDF pages 53-56).
[13] Ries, LAG., Eisner, MP., Kosary, CL., Hankey, BF., Miller, BA., Clegg, L and Edwards, BK. 2002. SEER Cancer Statistics Review 1973-1999: Overview in a Single PDF. National Cancer Institute. Bethesda, MD. Available online at http://seer.cancer.gov/csr/1973_1999/sections.html.
[14] Ries, LAG., Eisner, MP., Kosary, CL., Hankey, BF., Miller, BA., Clegg, L and Edwards, BK. 2002. SEER Cancer Statistics Review 1973-1999: Childhood Cancer by the International Classification of Childhood Cancer (ICCC). National Cancer Institute. Bethesda, MD. Available online at http://seer.cancer.gov/csr/1973_1999/sections.html.
[15] Ries, LAG., Eisner, MP., Kosary, CL., Hankey, BF., Miller, BA., Clegg, L and Edwards, BK. 2002. SEER Cancer Statistics Review 1973-1999: Sections of the CSR, 1973-1999, Testis. National Cancer Institute. Bethesda, MD. Available online at http://seer.cancer.gov/csr/1973_1999/sections.html.
[16] Environmental Protection Agency (EPA). 2003. Draft final guidelines for carcinogen risk assessment (external review draft, February 2003). Available online at http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=55445.
[17] DuPont Haskell Laboratory. 2002. Developmental and one-generation reproduction study: Mixture of poly(difluoro-methylene), alpha-fluoro-omega [2-(phosphonooxy) ethyl]-, monoammonium salt (CAS# 65530-71-4); poly(difluoro-methylene), alpha-fluoro-omega[2-(phosphonooxy) ethyl]-, diammonium salt (CAS# 65530-72-5); poly(difluoromethylene), alpha, alpha’- [phosphinicobis(oxy-2,1-ethanediyl)bis [omega-fluoro-], ammonium salt (CAS# 65530-70-3); isopropyl alcohol (CAS# 67-63-0); and water (CAS# 7732-18-5). US Environmental Protection Agency: Toxic Substance Control Act (TSCA) Section 8(e) Submission Received from 01/02/03 to 1/15/03: 8EHQ-1202-15247A. December 20, 2002. Available online at http://www.epa.gov/opptintr/tsca8e/doc/new8e.htm.
[18] DuPont Haskell Laboratory. 2002. Subchronic toxicity study: Mixture of poly(difluoro-methylene), alpha-fluoro-omega [2-(phosphonooxy) ethyl]-, monoammonium salt (CAS# 65530-71-4); poly(difluoro-methylene), alpha-fluoro-omega[2-(phosphonooxy) ethyl]-, diammonium salt (CAS# 65530-72-5); poly(difluoromethylene), alpha, alpha’- [phosphinicobis(oxy-2,1-ethanediyl)bis [omega-fluoro-], ammonium salt (CAS# 65530-70-3); isopropyl alcohol (CAS# 67-63-0); and water (CAS# 7732-18-5) (Telomer B Phoshate). US Environmental Protection Agency: Toxic Substance Control Act (TSCA) Section 8(e) Submission Received from 02/27/02 thru 03/13/02: 8EHQ-0202-15072A. February 6, 2002. Available online at http://www.epa.gov/oppt/tsca8e/doc/8esub/8e031302.htm.
[19] DuPont. 2002. The updated copy of DuPont Product Stewardship on December 17, 2001. U.S. EPA Administrative Record AR226-1069.
[20] DuPont Haskell Laboratory. 2002. Results of an oral gavage combined 90-day repeated dose and one-generation reproductive toxicity study in rats for poly (oxy-1,2-ethanediyl) alpha-hydro-omega-hydroxy- ether, with alpha-fluoro- omega (2-hydroxyethyl) poly (difluoromethane) (1:1) (telomer B monoether)(CAS Number 65545-80-4; non-HPV). US Environmental Protection Agency: Toxic Substance Control Act (TSCA) Section 8(e) Submission Received from 10/15/01 thru 12/07/01: 8EHQ-1001-14915. November 5, 2001. Available online at http://www.epa.gov/opptintr/tsca8e/doc/8esub/8e101501.htm.
[21] DuPont. 2002. DuPont flurotelomer product stewardship update, presented November 25, 2002. U.S. EPA Administrative Record AR226-1147.
[22] Hill, RN., Crisp, TM., Hurley, PM., Rosenthal, SL and Singh, DV. 1998. Risk assessment of thyroid follicular cell tumors. Environ Health Perspect 106(8): 447-57.
[23] Hurley, PM. 1998. Mode of carcinogenic action of pesticides inducing thyroid follicular cell tumors in rodents. Environ Health Perspect 106(8): 437-45.
[24] Olsen, GW., Burlew, MM., Burris, JM and Mandel, JH (2001). Final report: A longitudinal analysis of serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) levels in relation to lipid and hepatic clinical chemistry test results from male employee participants of the 1994/95, 1997, and 2000 fluorochemical medical surveillance program, 3M Medical Department, Epidimiology 220-3W-05.
[25] Olsen, GW., Burlew, MM., Burris, JM and Mandel, JH (2001). Final report: A cross-sectional analysis of serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to clinical chemistry, thyroid hormone, hematoloty and urinalysis results from male and female employee participants of the 2000 Antwerp and Decatur fluorochemical medical surveillance program, 3M Medical Department, Epidimiology 220-3W-05.
[26] Olsen, GW., Gilliland, FD., Burlew, MM., Burris, JM., Mandel, JS and Mandel, JH. 1998. An epidemiologic investigation of reproductive hormones in men with occupational exposure to perfluorooctanoic acid. J Occup Environ Med 40(7): 614-22. Also reviewed in U.S. EPA Administrative Record AR226-1137 (pages 147-149; PDF pages 44-46).
[27] Biegel, LB., Liu, RC., Hurtt, ME and Cook, JC. 1995. Effects of ammonium perfluorooctanoate on Leydig cell function: in vitro, in vivo, and ex vivo studies. Toxicol Appl Pharmacol 134(1): 18-25.
[28] vom Saal, FS., Timms, BG., Montano, MM., Palanza, P., Thayer, KA., Nagel, SC., Dhar, MD., Ganjam, VK., Parmigiani, S and Welshons, WV. 1997. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci U S A 94(5): 2056-61.
[29] DuPont (1997). Hazard characterization for human health C8 exposure CAS registry no. 3825-26-1. Prepared by L.B. Biegel, Senior Research Toxicologist.
[30] Hollowell, JG., Staehling, NW., Flanders, WD., Hannon, WH., Gunter, EW., Spencer, CA and Braverman, LE. 2002. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87(2): 489-99.
[31] Haddow, JE., Palomaki, GE., Allan, WC., Williams, JR., Knight, GJ., Gagnon, J., O'Heir, CE., Mitchell, ML., Hermos, RJ., Waisbren, SE., Faix, JD and Klein, RZ. 1999. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341(8): 549-55.
[32] Upham, BL., Deocampo, ND., Wurl, B and Trosko, JE. 1998. Inhibition of gap junctional intercellular communication by perfluorinated fatty acids is dependent on the chain length of the fluorinated tail. Int J Cancer 78(4): 491-5.
[33] Liu, RC., Hurtt, ME., Cook, JC and Biegel, LB. 1996. Effect of the peroxisome proliferator, ammonium perfluorooctanoate (C8), on hepatic aromatase activity in adult male Crl:CD BR (CD) rats. Fundam Appl Toxicol 30(2): 220-8.
[34] National Toxicology Program (NTP). 2002. 10th Report on Carcinogens. Available online at http://ehp.niehs.nih.gov/roc/toc10.html.
[35] Environmental Protection Agency (EPA). 2002. Revised draft hazard assessment of perfluorooctanoic acid and its salts, November 4, 2002. U.S. EPA Administrative Record AR226-1136.
[36] Thayer, KA., Ruhlen, RL., Howdeshell, KL., Buchanan, DL., Cooke, PS., Preziosi, D., Welshons, WV., Haseman, J and vom Saal, FS. 2001. Altered prostate growth and daily sperm production in male mice exposed prenatally to subclinical doses of 17alpha-ethinyl oestradiol. Hum Reprod 16(5): 988-96.
[37] Environmental Protection Agency (EPA). 2003. Preliminary risk assessment of the developmental toxicity associated with exposure to perfluorooctanoic acid and its salts. March 17, 2003.
[38] Godfrey, KM and Barker, DJ. 2001. Fetal programming and adult health. Public Health Nutr 4(2B): 611-24.
[39] Hales, CN and Barker, DJ. 2001. The thrifty phenotype hypothesis. Br Med Bull 60: 5-20.
[40] Eriksson, J., Forsen, T., Tuomilehto, J., Osmond, C and Barker, D. 2000. Fetal and childhood growth and hypertension in adult life. Hypertension 36(5): 790-4.
[41] Eriksson, JG., Forsen, T., Tuomilehto, J., Jaddoe, VW., Osmond, C and Barker, DJ. 2002. Effects of size at birth and childhood growth on the insulin resistance syndrome in elderly individuals. Diabetologia 45(3): 342-8.
[42] Eriksson, JG., Forsen, T., Tuomilehto, J., Osmond, C and Barker, DJ. 2000. Early growth, adult income, and risk of stroke. Stroke 31(4): 869-74.
[43] Eriksson, JG., Forsen, T., Tuomilehto, J., Winter, PD., Osmond, C and Barker, DJ. 1999. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. Bmj 318(7181): 427-31.
[44] Eriksson, JG and Forsen, TJ. 2002. Childhood growth and coronary heart disease in later life. Ann Med 34(3): 157-61.
[45] Ong, KK and Dunger, DB. 2002. Perinatal growth failure: the road to obesity, insulin resistance and cardiovascular disease in adults. Best Pract Res Clin Endocrinol Metab 16(2): 191-207.
[46] Stettler, N., Bovet, P., Shamlaye, H., Zemel, BS., Stallings, VA and Paccaud, F. 2002. Prevalence and risk factors for overweight and obesity in children from Seychelles, a country in rapid transition: the importance of early growth. Int J Obes Relat Metab Disord 26(2): 214-9.
[47] Palazzolo, M (1993). 13-week dietary toxicity study with T-5180, ammonium perfluorooctanoate (CAS N. 3826-1) in male rats. Report prepared for 3M, St. Paul, MN, Reviewed in US EPA "Draft PFOA Hazard Assessment" AR226-1079.
[48] Cook, JC., Murray, SM., Frame, SR and Hurtt, ME. 1992. Induction of Leydig cell adenomas by ammonium perfluorooctanoate: a possible endocrine-related mechanism. Toxicol Appl Pharmacol 113(2): 209-17.
[49] Butenhoff, J., Costa, G., Elcombe, C., Farrar, D., Hansen, K., Iwai, H., Jung, R., Kennedy, G, Jr.., Lieder, P., Olsen, G and Thomford, P. 2002. Toxicity of Ammonium Perfluorooctanoate in Male Cynomolgus Monkeys after Oral Dosing for 6 Months. Toxicol Sci 69(1): 244-257. Also reviewed in US EPA Reviewed in US EPA "Revised Draft PFOA Hazard Assessment-Robust Study Annex" AR226-1137, p. 244-253.
[50] Goldenthal, EI., Jessup, DC., Geil, RG and Mehring, JS. 1978. Ninety-day subacute rhesus monkey toxicity study: Fluorad ¨ Fluorochemical FC-143. Report prepared for 3M, St. Paul, MN by Institutional Research and Devlopment Corporation (Mattawan, MN). Study No. 137-090. Reviewed in US EPA "Draft PFOA Hazard Assessment" AR226-1079.
[51] Derelanko, MJ. 2000. Toxicologist Pocket HandbookWashington DC, CRC Press.
[52] Gilliland, FD and Mandel, JS. 1996. Serum perfluorooctanoic acid and hepatic enzymes, lipoproteins, and cholesterol: a study of occupationally exposed men. Am J Ind Med 29(5): 560-8. Reviewed in US Environmental Protection Agency Administrative Record AR226-1137 (pages 153-155; PDF page 50-52).
[53] Tomatis, L. 2002. The IARC monographs program: changing attitudes towards public health. Int J Occup Environ Health 8(2): 144-52.
[54] Melnick, RL. 2002. The IARC evaluation of di(2-ethylhexyl)phthalate (DEHP): a flawed decision based on an untested hypothesis. Int J Occup Environ Health 8(3): 284-6.
[55] Metrick, M and Marias, AJ (1977). 28-day oral toxicity study with FC-143 in albino rats. Final report. Report prepared for 3M, St. Paul, MN by Industrial Bio-Test Laboratories. Study No. 8532-10654, 3M Reference No. T-1742CoC, Lot 269., Reviewed in US EPA administrative record number AR226-1079.
[56] Perrone, CE., Shao, L and Williams, GM. 1998. Effect of rodent hepatocarcinogenic peroxisome proliferators on fatty acyl-CoA oxidase, DNA synthesis, and apoptosis in cultured human and rat hepatocytes. Toxicol Appl Pharmacol 150(2): 277-86.
[57] Huff, J. 2002. IARC monographs, industry influence, and upgrading, downgrading, and under-grading chemicals: a personal point of view. International Agency for Research on Cancer. Int J Occup Environ Health 8(3): 249-70.
[58] Sass, J. 2002. Lead IARC towards compliance with WHO/IARC Declaration of Interests (DOI) policy. Int J Occup Environ Health 8(3): 277-8.
[59] Baines, CJ. 2003. Transparency at the International Agency for Research on Cancer (IARC). Lancet 361(9359): 781-2.
[60] Kleihues, P. 2003. Transparency at the International Agency for Research on Cancer (IARC). Lancet 361(9359): 781.
[61] Burton, A. 2003. Is industry influencing IARC to downgrade carcinogens? Lancet Oncol 4(1): 4.
[62] No authors listed. 2003. Transparency at IARC. Lancet 361(9353): 189.
[63] Cimini, A., Cristiano, L., Bernardo, A., Farioli-Vecchioli, S., Stefanini, S and Ceru, MP. 2000. Presence and inducibility of peroxisomes in a human glioblastoma cell line. Biochim Biophys Acta 1474(3): 397-409.
[64] Yang, Q., Abedi-Valugerdi, M., Xie, Y., Zhao, XY., Moller, G., Nelson, BD and DePierre, JW. 2002. Potent suppression of the adaptive immune response in mice upon dietary exposure to the potent peroxisome proliferator, perfluorooctanoic acid. Int Immunopharmacol 2(2-3): 389-97.
[65] Yang, Q., Xie, Y., Alexson, SE., Nelson, BD and DePierre, JW. 2002. Involvement of the peroxisome proliferator-activated receptor alpha in the immunomodulation caused by peroxisome proliferators in mice. Biochem Pharmacol 63(10): 1893-900.
[66] Yang, Q., Xie, Y and Depierre, JW. 2000. Effects of peroxisome proliferators on the thymus and spleen of mice. Clin Exp Immunol 122(2): 219-26.
[67] Yang, Q., Xie, Y., Eriksson, AM., Nelson, BD and DePierre, JW. 2001. Further evidence for the involvement of inhibition of cell proliferation and development in thymic and splenic atrophy induced by the peroxisome proliferator perfluoroctanoic acid in mice. Biochem Pharmacol 62(8): 1133-40.
[68] Liu, RC., Hahn, C and Hurtt, ME. 1996. The direct effect of hepatic peroxisome proliferators on rat Leydig cell function in vitro. Fundam Appl Toxicol 30(1): 102-8.
[69] Berthiaume, J and Wallace, KB. 2002. Perfluorooctanoate, perflourooctanesulfonate, and N-ethyl perfluorooctanesulfonamido ethanol; peroxisome proliferation and mitochondrial biogenesis. Toxicol Lett 129(1-2): 23-32.
[70] Haughom, B and Spydevold, O. 1992. The mechanism underlying the hypolipemic effect of perfluorooctanoic acid (PFOA), perfluorooctane sulphonic acid (PFOSA) and clofibric acid. Biochim Biophys Acta 1128(1): 65-72.
[71] Van Rafelghem, M., Andersen, ME., Bruner, R and Mattie, D. 1982. Comparative toxicity of perfluoro-n-decanoic acid in rats and hamsters. Toxicologist 2: 148.
[72] George, ME and Andersen, ME. 1986. Toxic effects of nonadecafluoro-n-decanoic acid in rats. Toxicol Appl Pharmacol 85(2): 169-80.
[73] Kelling, CK., Van Rafelghem, MJ., Menahan, LA and Peterson, RE. 1987. Effects of perfluorodecanoic acid on hepatic indices of thyroid status in the rat. Biochem Pharmacol 36(8): 1337-44.
[74] Van Rafelghem, MJ., Vanden Heuvel, JP., Menahan, LA and Peterson, RE. 1988. Perfluorodecanoic acid and lipid metabolism in the rat. Lipids 23(7): 671-8.
[75] Gutshall, DM., Pilcher, GD and Langley, AE. 1988. Effect of thyroxine supplementation on the response to perfluoro-n- decanoic acid (PFDA) in rats. J Toxicol Environ Health 24(4): 491-8.
[76] Harris, MW., Uraih, LC and Birnbaum, LS. 1989. Acute toxicity of perfluorodecanoic acid in C57BL/6 mice differs from 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam Appl Toxicol 13(4): 723-36.
[77] Bookstaff, RC., Moore, RW., Ingall, GB and Peterson, RE. 1990. Androgenic deficiency in male rats treated with perfluorodecanoic acid. Toxicol Appl Pharmacol 104(2): 322-33.
[78] Davis, JW, 2nd., Vanden Heuvel, JP and Peterson, RE. 1991. Effects of perfluorodecanoic acid on de novo fatty acid and cholesterol synthesis in the rat. Lipids 26(10): 857-9.
[79] Nelson, DL., Frazier, DE, Jr.., Ericson, JE., Tarr, MJ and Mathes, LE. 1992. The effects of perfluorodecanoic acid (PFDA) on humoral, cellular, and innate immunity in Fischer 344 rats. Immunopharmacol Immunotoxicol 14(4): 925-38.
[80] Environmental Protection Agency (EPA). 2002. Annex of robust study Summaries from the revised draft hazard assessment of perfluorooctanoic acid and its salts, November 4, 2002. U.S. EPA Administrative Record AR226-1137.
Teflon and other non-stick pans kill birds
April 2003

Bird enthusiasts and veterinarians have known for decades that Teflon-coated and other non-stick cookware, if heated to high temperatures, is acutely toxic to birds. The peer-reviewed literature contains numerous reports of bird deaths linked to the use of Teflon and other non-stick pans and appliances in the home, beginning about 30 years ago. The birds die abruptly, usually shortly after new non-stick pans are heated for the first time. The ubiquity of the deaths has spurred mention of the problem on at least 100 websites devoted to the care of pet birds.
In 1975, in one of the early peer-reviewed articles on bird deaths, the authors describe the deaths of five pet birds following the owner heating a non-stick (PTFE-coated) pan:
“Five cocatiels (Nymphicus hollandicus) died within 30 minutes following exposure to fumes from a frying pan coated with the "non-stick" plastic polytetrafluoroethylene (PTFE) that had accidentally overheated. Within an hour the owner developed symptoms of "polymer fume fever" but recovered in the next 24 hours. Clinical signs and post mortem lesions of the cockatiels are described and reference is made to the unusual susceptibility of parakeets to the pyrolysis products of frying pans coated with PTFE.” [1]
Bird deaths related to nonstick coatings are not restricted to exotic species in the home. A recent article recounts that hours after moving 2400 broiler chicks to a research warehouse at University of Columbia-Missouri, veterinarians noticed that substantial numbers of chicks were dying. Four percent of the chicks died in the first four hours, and within 72 hours more than half of the chicks were dead. After investigating the possibility of many common gas toxicants, scientists traced the deaths to lightbulbs coated with the Teflon chemical PTFE: “Further investigation revealed that the only change in management practice in this facility prior to the onset of the severe mortality problem was the replacement of 48 heat lamp bulbs (one for each pen). The new heat lamp bulbs were polytetrafluoroethylene (PTFE) coated. PTFE gas intoxication has been reported in several exotic avian species, but this intoxication has not been previously reported in a poultry flock.” [2].
Scientists have not identified the particular offgas compound from Teflon and other nonstick pans and other kitchen equipment that is responsible for the bird deaths, but among the many chemicals that have been measured in the air when nonstick pans are heated are PFOA and other gases that scientists consider highly toxic [3].
References:
- Blandford TB, Seamon PJ, Hughes R, Pattison M, Wilderspin MP. 1975. A case of polytetrafluoroethylene poisoning in cockatiels accompanied by polymer fume fever in the owner. Vet Rec 1975 Feb 22;96(8):175-8.
- Boucher M, Ehmler TJ, Bermudez AJ. 2000. Polytetrafluoroethylene gas intoxication in broiler chickens. Avian Dis 2000 Apr-Jun;44(2):449-53.
- Ellis DA, Mabury SA, Martin JW, Muir DC. 2001. Thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment. Nature 2001 Jul 19;412(6844):321-4.
Related websites:
upatsix.com
Description: Up At Six is a very comprehensive pet bird care website. Lists common household hazards and provides a search engine for research by topic or keywords. Also links to avian associations, aviaries, sanctuaries, shopping, online chat groups, and many other bird-related issues.
Association of Avian Veterinarians: Basic Bird Care
http://www.aav.org/basic_care.htm#Avoid
Description: Association of Avian Veterinarians promotes avian medicine and stewardship. Become a member, find an avian vet, learn about AAV programs and policy statements.
PetPlace.com article: Dangers in the Kitchen
http://www.petplace.com/articles/artShow.asp?artID=3534
Description: PetPlace.com offers access to a wealth of medical and wellness pet information. Features an archive of more than 1350 pet-related articles searchable through a user-friendly database.
The National Cockatiel Society: General Bird Care
http://www.cockatiels.org/articles/care/gencare.html
Description: The official website of the National Cockatiel Society. Features a question and answer bulletin board with scheduled chats and “reading rooms” of cockatiel-related articles.
Planet-Pets.com: Housing/Feeding Birds
http://www.planet-pets.com/feedbrd.htm
Description: Planet Pets serves the public by finding, evaluating, selecting, organizing, and offering quality pet-related information and resources. Features a free online newsletter, kids korner, ask an expert, links to services.
Birds and More
Health issues and
Protecting birds from toxic fumes
Description: Online store for birds and their owners. Includes health tips and general care for your bird.
TalkToTheVet - Caged Bird Housing Considerations
http://www.talktothevet.com/ARTICLES/BIRDS/Pbirdhousing.HTM
Description: Talk To The Vet is an online veterinarian consultation service. Links to vets, free newsletter, pet-specific articles, etc.
Bird Crazy - Safety Guide for Plants and Poisons
http://www.birdcrazy.com/newsite/articles/plants.htm
Description: Bird Crazy is an Online aviary based in San Diego, CA. Comprehensive list of poisonous plants and household poisons and well as a list of safe plants.
Birds N' Ways eZine
http://www.birdsnways.com/wisdom/ww1e.htm
Description: Guide to parrots and other exotic pet birds. Offers dozens of links to shopping, chatting, information and miscellaneous bird-related sites. Offers access to thousands of articles searchable by keyword, topic, author and website.
ASPCA - Managing Pet Bird Toxicoses
http://www.aspca.org/site/Search?query=PTFE&inc=10
Description: American Society for the Prevention of Cruelty to Animals exists to promote humane principles, prevent cruelty and alleviate fear, pain and suffering in animals. Use the search engine (search “PTFE”) to find a downloadable report “Managing Pet Bird Toxicoses”.
Additional Links:
Starling Talk
http://www.starlingtalk.com/warning.htm
Parrot Parrot
http://www.parrotparrot.com/birdhealth/kola.htm
Exotic Pet Vet
http://www.exoticpetvet.net/about.html
Kaytee
http://www.kaytee.com/companion_animals/birds/health/
PETA
http://www.petauk.org/mc/facts/fsc16.html
Pet Bird Report
http://www.petbirdreport.com/articles/dangers.html
The Parrot Chronicles
http://www.parrotchronicles.com/departments/hazards.htm
Feather Care Aviary
http://home.cogeco.ca/~jjackson283/index.htm
The Parrot Society of Australia
http://www.parrotsociety.org.au/
Stanley’s Quakerville
http://www.quakerville.com/features/ptfe.asp
Lafeber Company
http://www.lafeber.com/docs_book/13/thirty_three_dangers.htm
The Aviary
http://theaviary.com/teflon.shtml
Avitec Exotic Birds
http://www.avitec.com/Birdcare.html
Budgie.org
http://www.budgies.org/info/teflon.html
Those Majestic Macaws!
http://www.exoticbird.com/teflon.html
Taking Care Of Your New Bird
http://www.ddc.com/~kjohnson/safety.htm
Lovebird Center
http://www.lovebirdcenter.com/7hazards.htm
Cockatiel Cottage
http://www.cockatielcottage.net/hazards.html
New England Exotic Bird Sanctuary
http://www.neebs.org/poisons.htm
South Bay Bird Society
http://www.sobaybirdsoc.com/hazards.htm
Austin Bird Club
http://www.austinbirdclub.com/dangerous.htm
Old World Aviaries
http://www.oldworldaviaries.com/text/styles/teflon.html
http://www.oldworldaviaries.com/text/miscellaneous/buying_a_bird.htm
Bignest Aviaries
http://www.bignest.com/food.htm
http://www.bignest.com/misgrey.htm
Wee B Toys
http://www.weebbirds.com/firstaid.htm
Pet Bird Care Safety Tips and Information
http://members.tripod.com/~DAdams/qkbrdinf.html
Parrot Online
http://www.parrotline.org/teflon.html
Bella Online
http://bellaonline.com/articles/art3973.asp
Senegal
http://www.makasnest.com/senegal/Teflon/toxins.htm
Feathers and Tails
http://www.feathersandtails.org/holiday.html
Birds By Donna
http://www.birdsbydonna.com/BIRDS_BY_DONNA/Basic_Bird_Cax.html
Kindness Club
http://www.kindnessclub.nb.ca/pets/BirdSafety.html
Rockport Roost
http://www.rockportroost.com/Birdsafetytips.htm
Animal Lovers Pet Shop
http://www.animalloverspetshop.com/whatsnew.htm
The Real Macaw
http://www.realmacaw.com/pages/tielcare.html
Wool-N-Wings
http://www.parrots.com/faq-hn.htm
African Lovebird Society
http://www.africanlovebirdsociety.com/lovebirdcare/
North American Cockatiel Society
http://www.cockatiel.org/articles/hazards.html
Angel Wings Aviary
http://www.parrot-breeder.com/parrothazards.htm
A&M Aviaries
http://www.petbirdbreeder.com/hazards.htm
The Animal Clinic of Clifton
http://www.pethealthcare.net/html/body_household_dangers.html
Birdsafe.com
http://www.birdsafe.com/story_bird33.htm
NetPets
http://www.netpets.org/birds/healthspa/poisons2.html
Arizona Avian Breeders Association
http://azavianbreeders.org/BirdCare.shtml
Avian Avenue
http://zachary.avianavenue.com/BirdSafety.html
Ask Dr. Petra
http://www.drpetra.com/Birds/Household%20Dangers.htm
Long Island Parrot Society of New York
http://www.liparrotsociety.org/harmful.htm
http://www.liparrotsociety.org/teflon!.htm
The Grey Place
http://www.africangreys.com/hints/emergency.htm
Birds Birds Birds!
http://parrotfeathers.tripod.com/care.htm
The Family Pet Show
http://www.familypetshow.com/toxocosis.html
Doctors Foster and Smith
http://www.drsfostersmith.com/Articles/bird_commoninjuries.cfm
Parrots Canada
http://www.parrotscanada.com/birdowner/forthenewbirdowner.html
Birds Just Wanna Have Fun
http://www.birdsjustwannahavefun.com/durability_info.htm
American Animal Hospital Association
http://www.healthypet.com/Library/prevent-18.html
Soldan’s Feed and Pet Supplies
http://www.soldanspet.com/petperspectives/bird_basics.htm
British Columbia SPCA
http://www.spca.bc.ca/Factsheets/birdcare.htm
The Avian Medicine Chest
http://www.petmedicinechest.com/avian/discussions/chemicalpoisoningtext.asp
Pet Parrot Club UK
http://www.petparrotclubuk.co.uk/help/hazards.htm
The Pet Bird Website
http://www.petbird.co.uk/housedangers.htm
Quaker Parakeet
http://www.quakerparakeet.com/b4ubuy.html
Houston Pet Talk
http://www.houstonpettalk.com/Column_Bird_Buddies.htm
Hex Aviary
http://www.hexaviary.com/hexlocal.htm
Birds of a Feather
http://www.boaf.com/article_11.htm
All Creatures Animal Hospital
http://www.all-creatures.com/care/bird.htm
Feathered Friends Forever
http://www.featheredfriendsforever.org/homesafety.html
Bird Crazy
http://www.birdcrazy.com/toxins.htm
The Parrotlet Parlour
http://www.theparrotletparlour.com/Parrotlet_Hazards.shtml
The Windhover Veterinary Center
http://www.windhovervet.com/birdcare.htm
Arizona Bird Supply
http://www.azbirdsupply.com/bird%20care_basics.htm
The PetCARE Information Center
http://petcare.umn.edu/OPets/Articles/BirdTox.htm
The Holistic Bird Newsletter
http://www.holisticbirds.com/HBN01/octnov/pages/chronic1.htm
Win Rose Animal Hospital
http://www.winrosevet.com/Birds.htm
Harmony’s World of Pets
http://www.petvets.com/petcare/birdscolumn.html
Janice’s Bird Haven
http://www.wtv-zone.com/JaniceMarr/feathered/Care.html
The Bird Hotline
http://www.birdhotline.com/vet.htm
The True Parrot
http://thetrueparrot.homestead.com/EnvironmentalNeeds.html
The Grey-cheeked Parakeet
http://www.lib.montana.edu/~bmarsh/cheeky/dosdonts.html
Dandy the African Grey Parrot
http://www.vdnent.com/html/articles/part_behav_be-a-bird.htm
Quaker Parrots
http://www.quakerparrots.com/qtips/more_thoughts_on_teflon.htm/
http://www.quakerparrots.com/qtips/dangers_of_teflon.htm
Animal Rescue League of Iowa
http://www.arl-iowa.org/PetCare/Birds.asp
Wasatch Avian Education Society
http://www.wasatchavian.com/fosterguide.html
Dane County Humane Society
http://www.humanesociety.dane.wi.us/care/bird_groom.html
Montreal Bird and Exotic Animal Hospital
http://www.birdandexoticvet.com/avian.html
Parrot Passions UK
http://www.parrotpassionsuk.com/Advice/hazards.htm
Birdlife
http://www.birdlife.com/care/dangers.html
Pretty Birds
http://www.prettybirds.net/Parrotsafehome.htm
The Animal Network
http://www.animalnetwork.com/birds/library/articleview3.asp?Section=&RecordNo=2721
Pet Finder
http://www.petfinder.org/journalindex.cgi?path=/public/animalcare/bird/1.3.404.txt
Budgies ’N Tiels
http://www.budgies-n-tiels.com/contents/health.html
Pair o' Keets and Qweak n' Tails
http://www.geocities.com/monicaarnouk/BirdHazards.htm
SunConure
http://www.sunconure.com/safety.html
Give Us A Home
http://www.giveusahome.co.uk/birds/faq.htm
Eve’s India
http://www.evesindia.com/family/pets/bird_safety.html
MSN Health
http://content.health.msn.com/content/article/35/1728_84136
Pet Education
http://www.peteducation.com:80/article.cfm?cls=15&cat=1829&articleid=2418 http://www.peteducation.com:80/article.cfm?cls=15&cat=1912&articleid=1559
North Fork Animal Hospital
http://www.northforkanimalhospital.com/animals/birds/birds_quaker.htm
Regulatory gaps lead to global PFC pollution
April 2003
Although most people assume that chemicals in consumer products are thoroughly tested before they are sold, there is no legal requirement to test most chemicals for health effects at any stage of production, marketing, and use. Because of this, basic toxicology studies on Teflon, Scotchgard and related chemicals are being conducted only now, fifty years after these chemicals went on the market.
Under the Toxic Substances Control Act, chemical companies can continue making chemicals and putting new compounds on the market without conducting any studies of their effects on people or the environment. Some companies conduct rudimentary screening studies prior to production, but fewer than half of all applications to the EPA for new chemical production include any toxicity data at all. The government approves 80 percent of these applications with no restrictions, usually in less than three weeks. When data are provided, they are typically cursory in nature, because the government lacks authority to request anything more than that. Eight of 10 new chemicals win approval in less than three weeks, at an average rate of seven a day. If there are no data, the government justifies approval with results of computer models that estimate if a chemical will harm human health or the environment (EPA 1997a, GAO 1994).
Chemicals are stuided after the damage is done
For chemicals that are already on the market, the EPA can request data only when it can substantiate that the chemical is causing harm, which it generally cannot do without the toxicity data it is seeking to request. In practice, this means that studies are required only after independent scientists have accumulated a body of evidence demonstrating potential harm, a process that typically takes decades.
In general, the more recently a chemical has been introduced into commerce, the less scientists understand its toxicity, and the less likely it is that scientists will know how to test for it in people and the environment. The few chemicals or chemical families that have been well-studied are those for which scientists uncovered, often accidentally, environmental catastrophes that can include widespread pollution of the environment or human population, as is the case now for PFCs.
Part 9: Sidebars
Sidebar 1: Reform of Federal Law
Sidebar 2: The regulatory precedent of pesticides
Reform of federal law
April 2003
Imagine a regulatory system designed in theory to protect hundreds of millions of people from the potential harm of tens of thousands of chemicals in products they use every day. Imagine that this system did not require any health or safety studies prior to the marketing and sale of a chemical; did not require any monitoring of chemicals once they were in use; allowed producers to claim virtually all information related to a chemical as confidential business information and thus forever shield it from public view; and did not allow the public any right to sue or otherwise force testing or monitoring when independent scientists confirmed that significant contamination or hazards may exist.
That is the reality of the Toxic Substances Control Act, the nation’s chief regulatory statute for commercial chemicals. TSCA, as it is known, is famous for the lack of authority it provides the Environmental Protection Agency. Under TSCA a chemical company is under no legal obligation to understand how its products might harm human health. And in fact, only after scientists have amassed a body of evidence linking the chemical to human harm can the federal government ban it or leverage a phase out. A string of Congressional hearings and reports from the General Accounting Office have thoroughly documented this fact. With no statutory power to request data on a chemical prior to proving harm, which it typically cannot prove without the data it is seeking, the EPA has essentially given up trying to use TSCA to better understand the potential hazards of the tens of thousands of chemicals in use today.
More than 63,000 chemicals were granted blanket approval for use in consumer and industrial products with the passage of TSCA in 1976. The federal government reviews the safety of chemicals invented since that time through an application process that does not require health and safety test data and that discourages voluntary testing. Companies submit basic toxicity data with fewer than half of all applications to manufacture new chemicals; the government approves 80 percent of these with no restrictions and no requests for tests. Eight of 10 new chemicals win approval in less than three weeks, at an average rate of seven a day.
Companies can volunteer any studies they may have performed to files and dockets maintained by the Environmental Protection Agency, but in the absence of any voluntary submissions, EPA is forced to rely on computer models to estimate if an industrial chemical might be toxic to humans.
In 1998 EPA found that chemical manufacturers had failed to volunteer even the most basic information on chemical properties or toxicity for an estimated 43 percent of the 2800 chemicals produced in the highest quantities in the U.S. (EPA 1998b). A voluntary testing program grew out of this finding. Under this program, called the High Production Volume chemical testing program, or HPV program, participating companies submit their interpretation (but not the data) of eighteen basic screening tests, only one-third of which are directly relevant to human health and none of which include even a standard two-year cancer study, or tests for birth defects linked to low doses.
The group that leads the federal government’s efforts to assess testing needs on the health effects of industrial chemicals, the Interagency Testing Committee, or ITC, recently identified through the use of computer models 392 industrial chemicals expected to build up in the human body for which EPA lacks basic data from the manufacturers on chemical properties, uses, and toxicity. Among these are chemicals used in fragrances, dyes and pigments, polyurethane foam, and pesticides. There is no plan to study the presence of these chemicals in humans.
In effect, the nation has no regulatory system for chemicals that are not directly added to food (pesticides and food additives). Instead, we have a shell of a program that by law has weak authority to study, much less restrict the use of chemicals in commerce.
This statutory void has produced:
- Widespread, pervasive contamination of the human population with hundreds of chemicals at low dose mixtures that have never been examined for any of their potential health effects.
- An industry that has no legal obligation to conduct safety tests or monitor for the presence of its chemicals in the environment or the human population – and a significant financial incentive not to do so.
- A federal research establishment that is completely unequipped, both technically and financially, to monitor the human population for commercial chemicals or to study their health effects.
- An ever increasing load of chemical contamination in the human population and global environment that is comprised almost entirely of poorly studied chemicals that have never before been encountered in all of evolutionary history.
The chemical industry and its supporters argue that the suite of industrial chemicals found in an individual’s bloodstream is safe, and account for negligible increased health risks. The doses, they say, are too low to cause harm.
But there is no science to support this assertion.
The truth is that nobody knows the effects of the low dose mixtures of chemicals identified in this study, and the hundreds of other chemicals that are certain to be present in the body, but for which we could not test. Federal law imposes few health and safety testing requirements on the chemical industry, and sets few public health goals for chemical exposure or use.
Instead, industry decides what tests are done, when they are done, what the results mean, and who gets to see them. Overall, this system has left a void of scientific knowledge on the health and environmental hazards of nearly all chemicals found in consumer products and in people.
Safety margins erode further - new chemicals are invented daily.
The chemical industry gains permission to put more than 2000 new chemicals into the biosphere each year, with no knowledge of the health impacts on the exposed human population. People are given no warning of this exposure nor do they have the option to not be exposed.
The predictable outcome of this arrangement is that the dangers of chemicals are discovered only after widespread exposure and harm has occurred. The more recently a chemical has been introduced into commerce, the less scientists understand its toxicity, and the less likely it is that scientists will know how to test for it in people and the environment. New chemicals enter the marketplace with no, or only a handful of, toxicity studies. The few chemicals or chemical families that have been well-studied are those for which scientists uncovered, often accidentally, catastrophes or widespread contamination. For instance, the earnest study of DDT toxicity did not start until the discovery that the chemical was driving into extinction a number of bird species, including bald eagles. Intense research on the toxicity of perfluorinated chemicals is beginning only now, after 3M discovered that these Scotchgard ingredients, in use for 50 years, have broadly contaminated humans and are more toxic than previously believed.
And even for the best-studied chemicals, scientists have yet to gain a full understanding of health effects. When setting safety standards for electrical insulators called PCBs, banned in the U.S. since the 1970s, the World Health Organization reviewed 1,200 studies on PCB’s harmful effects and properties, but found only 60 that were relevant. In a similar review of PCBs the U.S. government enumerated 14 categories of uncertainty encompassing every step from human exposure to manifestation of health effects (EPA 1996). PCBs are among the best-studied chemicals in the world.
Chemical companies are not required to develop or divulge methods to test for the presence of their chemicals in the environment or the human body. Typically, only after a compound has been on the market for decades and contaminated a significant portion of the environment do independent scientists learn how to detect and quantify it. At that point, the Centers for Disease Control and Prevention (CDC) may choose to test for it in the general population, but even then there is no guarantee that the manufacturer will provide CDC with the methodology to detect it, or that the methods will be reliable. For instance, three years after 3M announced that it was removing the principle perfluorinated compound, PFOS (Scotchgard), from the market, chiefly because it contaminated the blood stream of the entire human race, the CDC still does not have a test method that it considers reliable to find the chemical in human blood.
Ignorance by Design
Detailed analyses by the U.S. EPA (EPA 1998b) and Environmental Defense (ED 1998) make clear how few health effects studies are available even for chemicals produced in the highest volumes. In a review of all publicly available toxicity and environmental fate studies, they found no information – not a single test - for 43 percent of the 2600 chemicals produced in the highest volumes in the US, with yearly production volumes of more than 1,000,000 pounds. Our study offers stark confirmation: for 55 compounds found in the nine individuals tested (one third of the chemicals identified), there is no information available - on chemical uses or health effects - in any of the eight standard industry and government references used for this analysis.
The work by the EPA and ED was important in establishing a baseline measure of data availability. But the tallies in the EPA and ED reports are only as meaningful as the studies they are counting. Both analyses focus on a limited universe of toxicity screens that themselves are not detailed enough to support regulation, and are not targeted toward the most meaningful and relevant health effects. But far worse than the numbers is the policy outcome that these analyses produced: a voluntary program for industry to conduct hundreds more of these same toxicity screens.
Launched with much fanfare in 1999, the so-called high production volume chemical screening, or HPV program, has not yielded data for EPA to review. Instead chemical manufacturers are submitting summaries of the screening studies, leaving EPA and the public at the mercy of industry’s interpretations of the data, which are not subject to independent peer review. The program is voluntary, and the EPA is powerless to demand any additional information. At the same time the HPV program provides invaluable public relations cover for the chemical manufacturers in the form of thousands of “studies” being conducted “voluntarily” at “great expense.”
And even if the actual screening study data were submitted, much of it would be of limited use. Consider the so-called cancer screens. In reality, what industry calls a cancer screen for public relations purposes under the HPV program, is nothing but a mutagenicity assay in a lab dish that both industry and regulators routinely dismiss as inconclusive in the absence of two-year animal studies confirming a carcinogenic effect.
Scientists often study the wrong thing
The nature of our ignorance of chemical exposure is more complicated than tallies of study numbers can convey. There are fundamental problems with even the best regulatory study methodologies when they are applied to the body burden of chemicals identified in this study. The vast majority of toxicity tests required by government regulators have limited relevance to the exposures that are occurring in the human population.
In a typical animal study required by the EPA, scientists test a single chemical in adult animals at high doses. The outcomes analyzed can include increases in the occurrence of tumors, changes in organ weight, or visible birth defects. Scientist don’t typically look for functional changes in response, such as brain development, following developmental exposure. Required developmental toxicity studies do not evaluate development after birth and tend to be less sensitive than studies that do assess postnatal function. A 1998 EPA draft report titled “A Retrospective Analysis of Twelve Developmental Neurotoxicity Studies Submitted to the USEPA Office of Prevention, Pesticides and Toxic Substances (OPPTS)” found that the developmental neurotoxicity study resulted in a lower “no observed effect level” for 10 of 12 chemicals compared to the required developmental rat studies that did not look at brain development (Makris, et al. 1998). The developmental neurotoxicity test is not a required test. EPA has requested it for only a small number of chemicals.
In contrast to high dose regulatory studies, people are exposed to multiple chemicals, from conception to death, at relatively low doses. The effects that occur can be subtle, detected across the general population as slight drops in IQ or fertility, or increases in specific types of cancer.
Some scientists, particularly those employed by the chemical industry, argue that “the mere presence” of small amounts of hundreds of chemicals in your bloodstream is biologically insignificant. High dose animal studies are typically offered as proof of this assertion. The truth, however, is that high dose animal studies cannot prove or disprove the safety of chemical exposures at lower doses, particularly when these studies are conducted primarily on adult animals, do not look for health endpoints relevant to low dose exposures, and do not account for interactions with other chemicals to which people are routinely exposed.
Industry’s dogmatic allegiance to the high dose theory of toxicology can be traced to the 16th century philosopher Paracelsus, whose philosophy is summarized in the well-known adage “The dose makes the poison.” The scientific and regulatory infrastructure in the US is based on studies that feed animals high doses of chemicals in the belief that a high dose will elicit any and all toxic effects that a compound can produce. In practice, if a high dose doesn’t elicit a readily measured toxic effect, then industry argues and regulators assume that the substance is not toxic. We now know that this is not true.
Science has advanced in the past 500 years, and outside of regulatory toxicology it is generally accepted that other factors besides dose — most notably the timing of the dose — are as important in determining the toxic effect.
The most obvious example is fetal exposure, where exposure in utero can produce long lasting adverse effects at amounts that produce no observable effects in adults. This outcome is documented in the scientific literature for lead, mercury and PCBs, where exposures in the parts per billion range in the womb or during infancy can lower IQ’s or alter behavior, while the same dose produces no observable effects in an adult. Dioxin is another case in point. Men with 80 parts per trillion of dioxin in their blood father nearly twice as many girls as boys. This effect would not have been predicted based on studies of adults.
Policy Recommendations
TSCA reform
Seven chemicals or chemical classes have been regulated or banned under the Toxic Substances Control Act (TSCA). When compared to the 75,000 chemicals registered for commercial use, the impact of TSCA is nearly imperceptible in the overall context of human chemical exposure. It is little wonder that the chemical industry considers TSCA the only truly workable federal environmental law.
Under TSCA, chemicals are assumed safe until they are proven hazardous. At the same time, the law does not require that manufacturers conduct health and safety studies, nor does it impose a duty on manufacturers to monitor how their products are used or where they end up in the environment.
As a starting point for a major environmental statute, this is problematic.
TSCA puts the burden of proving a chemical’s hazards squarely on the shoulders of the EPA (section 4 (1)(A)). The statute then prohibits the EPA from requiring safety tests unless the agency can prove that the chemical presents an unreasonable risk – which it can almost never prove because it cannot require the studies needed to make that finding. If the agency assembles enough data to require industry to conduct safety studies, it must go through the lengthy process of promulgating a test rule, very similar to a regulatory rule making, to mandate even one test for one chemical. When the data are generated, industry can claim the tests as confidential business information or trade secrets, and thus shield the tests from independent peer review or public scrutiny.
This law is so fundamentally broken that the statute needs to be rewritten. Revisions to the nation’s toxic substance laws must include the following provisions:
- For chemicals currently manufactured and used commercially, the chemical industry must submit to EPA all internal studies on the properties, environmental fate, potential human exposure pathways and exposure levels, concentrations in workers and the general population, levels in the environment, worker and community health, measured effects in wildlife, toxicity, mechanisms of action and any other information relevant to human exposures and potential health effects. These studies must be made available to the public.
- Industry must be required to prove the safety of a new chemical before it is put on the market.
- The EPA must have the unencumbered authority to request any and all new data on a chemical that is already on the market.
- The EPA must have the clear authority to suspend a chemical’s production and sale if the data requested are not generated, or if they show that the chemical, as used, is not safe for the most sensitive portion of the exposed population.
- Chemicals that persist in the environment or bioaccumulate in the food chain must be banned. Currently EPA cannot demand the data needed to make this determination, and industry is not volunteering it.
- Chemicals found in humans, in products to which children might be exposed, in drinking water, food, or indoor air, must be thoroughly tested for their health effects in low dose, womb-to-tomb, multi-generational studies focused on known target organs, that include sensitive endpoints like organ function and cognitive development. Studies to define mechanisms of action (how a chemical harms the body) must be conducted.
- The chemical industry must develop and make public analytical methods to detect their chemicals in the human body, and conduct biomonitoring studies to find the levels of their chemicals in the general population.
- Chemical manufacturers must fully disclose the ingredients of their products to the public.
The regulatory precedent of pesticides
April 2003
At first blush these statutory changes appear a radical departure from current policies, but in fact, the chemical industry already complies with these standards for pesticide products, proof that the industry can meet the same safety standards with commercial chemicals.
Pesticides in food are regulated under section 408 of the Food Drug and Cosmetic Act, which requires chemical companies to show that there is a “reasonable certainty of no harm” from exposure to a pesticide, for all exposed individuals, including explicit consideration of the fetus, infant and small child. This standard, which is well defined in case law and regulations, applies to all uses and all routes of exposure to a pesticide (food, air, and water considered together). “Reasonable certainty of no harm” is protective of the public health, particularly where the finding is contingent on fetal and infant exposure, but is not so protective that it cannot be met, or that companies can argue that it is onerous.
Section 408 also requires that pesticides with common mechanisms of toxicity be added together when assessing compliance with the reasonable certainty of no harm standard. This means that groups of pesticides, for example, all organophosphates, are added together when measuring compliance. In contrast, TSCA does not require that regulators assess the additive risks. Many major chemical classes commonly used in consumer products are characterized by common mechanisms of toxicity - phthalates, perfluorinated chemicals, and polybrominated diphenyl ethers, for example - and none are assessed in aggregate by EPA.
When data are not available, legal exposures for infants and children are required to be 10 times lower than for adults, and economic benefits are not allowed as an escape valve, or a means to permit higher risk.
To ensure that these tough standards can be met, the other governing statute, FIFRA (the Federal Insecticide Fungicide and Rodenticide Act), grants the EPA administrator broad (virtually unlimited) authority to request data, and to suspend the sale of the product when data are not generated (section 3, particular 3(c)2(B), and section 6). This is the key reform.
The legislative history of FIFRA is instructive. Beginning in the early 1980’s a series of congressional committee investigations and GAO reports documented that basic health studies had not been conducted for most pesticides on the market at that time. In response, Congress amended FIFRA in 1988 to require that all pesticides be “reregistered,”which meant that they had to be tested by contemporary standards and re-evaluated for their health risks.
This forced the EPA to deal with the same problem that they face today when considering a comprehensive testing program for toxic chemicals: what to do with all the chemicals already on the market?
EPA’s response, which largely was successful, albeit slow, was to impose strict timelines for testing and re-evaluation while granting EPA clear authority to require any test for any pesticide, and the authority to suspend the sale of a pesticide if the manufacturer refuses to do the test or fails to submit it on time. Compare this with TSCA where EPA must go through a rulemaking just to get one test on one chemical.
As a result of these amendments, EPA now requires about 120 tests for pesticide registration. These tests range from acute and chronic toxicity, to metabolism, environmental fate and residue chemistry. These tests include toxicity tests that will support regulatory decision making, not the superficial screening tests being conducted under the HPV testing program. EPA has reevaluated nearly all pesticides of any significance, starting in the early 1990’s with more than 100 pesticide active ingredients in about 20,000 different products applied to food crops. There is no reason that these same test requirements could not be applied in a tiered fashion to commercial chemicals regulated under TSCA.
Testing requirements alone have driven many compounds from the market. One good example is methoxychlor, a DDT relative, which was banned with little fanfare in 1999 when the manufacturer simply refused to conduct required health studies. Another good example is pesticides used in aircraft cabins. In 1995 EPA asked all manufacturers of pesticides applied inside commercial airplanes to do the exposure studies needed to show the use was safe. Not a single manufacturer of more than 200 products was willing to do the tests (because they knew that the use was not safe), and all uses of pesticides inside aircraft were unceremoniously banned in the United States in 1998.
Another great example of the power of FIFRA’s data generation authority involves the toxic byproducts of chlorinating tap water. The Safe Drinking Water Act does not give the EPA authority to require toxicity tests for drinking water contaminants. As a result, the agency is forced to negotiate test programs with polluters or the affected industry, or to pay for the testing from their own research funds. But because chlorine is a pesticide (it kills microbes in water), EPA was able to use the data call-in authority of FIFRA to require the chlorine industry to do a broad range of toxicity tests on chlorination byproducts that they otherwise had not planned to do.
Recommendations for Action on PFCs
April, 2003
What should the government do?
What should the chemical industry do?
What should the government do?
The unique combination of prevalence, toxicity, and persistence that characterizes chemicals in the PFC family argues for expedited action by the Environmental Protection Agency (EPA) to mitigate human exposures. We recommend that:
- EPA should require the phase-out of PFOA and industrial chemicals that break down into PFOA in the environment or the human body.
- EPA should expedite the review of all remaining PFCs and polyfluorochemicals, including fluoropolymers, and require the phase-out of those that are persistent or that break down into persistent PFCs.
- EPA should fully assess human health risks across the family of PFCs, considering the combined health effects of chemicals that exhibit common health harms and modes of action.
- CDC (Centers for Disease Control and Prevention) should adopt and earlier EWG proposal for long-term biomonitoring of PFCs in human blood to assess trends in exposure, which could increase for some PFCs even after a global ban, as the PFC family breaks down to its terminal degradation products (PFOA, PFOS, and others).
PFCs are highly toxic, extraordinarily persistent chemicals that pervasively contaminate human blood and wildlife the world over. This chemical tragedy has happened in large part because of a regulatory system that leaves the EPA with few tools to identify emerging pollution problems, or to anticipate and study the health effects or the extent of human exposure to any of the thousands of chemicals found in consumer products, including PFCs.
The Toxics Substances Control Act is so fundamentally broken that the statute needs to be rewritten. Revisions to the nation's toxic substance laws must include the following provisions:
- Industry must be required to prove the safety of a new chemical before it is put on the market.
- The EPA must have the unencumbered authority to request any and all new data on a chemical that is already on the market.
- The EPA must have the clear authority to suspend a chemical's production and sale if the data requested are not generated, or if they show that the chemical, as used, is not safe for the most sensitive portion of the exposed population.
- Chemicals that persist in the environment or bioaccumulate in the food chain must be banned.
- Chemicals found in humans, in products to which children might be exposed, in drinking water, food, or indoor air, must be thoroughly tested for their health effects in low dose, womb-to-tomb, multi-generational studies focused on known target organs, that include sensitive endpoints like organ function and cognitive development. Studies to define mechanisms of action (how a chemical harms the body) must also be conducted.
What should the chemical industry do?
- The chemical industry must phase out the use of PFOA and other chemicals that break down to PFOA in the environment or the human body, including uses in Teflon, Stainmaster, and Zonyl paper protection products.
- The chemical industry must develop and make public analytical methods to detect all PFCs in the human body, and conduct biomonitoring studies to find the levels of their chemicals in the general population.
- The chemical industry must disclose the breakdown products and environmental persistence of all PFCs, including perfluoro- and polyfluoro- polymer products like Gore-Tex, and discontinue the manufacture of those that are persistent.
- Chemical manufacturers must fully disclose the PFC ingredients of their products to the public.
Although some exposures to PFCs are unavoidable - they have been found in food, air, and drinking water - you can choose to avoid many PFCs in future purchases of consumer products. Doing so will help reduce the impacts of the "PFC" economy on human health and wild animals. Here are some tips:
- Phase out the use of Teflon and other non-stick cookware and other equipment that is heated in your home. If you can afford to replace it now, do so. When heated to high temperatures, Teflon and produccts with other non-stick PFC coatings emit fumes that can be harmful.
- Do not use Teflon or non-stick cookware in your home if you have pet birds. In fact, avoid any kitchen equipment that contains Teflon or other non-stick components that are heated to high temperature during use. Fumes from these materials can quickly kill birds.
- When you purchase furniture or carpet, decline optional treatments for stain and dirt resistance, and find products that have not been pre-treated with chemicals by questioning the retailers. Most of these chemical treatments contain PFCs that might contaminate your home and family.
- Avoid buying clothing that bears a Teflon label or other indication that it has been coated for water, stain, or dirt repellency. Many of these coatings are PFCs. By buying alternatives you will help shrink the PFC economy and the associated global contamination.
- Minimize packaged food and greasy fast foods in your diet. These can be held in containers that are coated with PFCs to keep grease from soaking through the packaging. PFCs are used in a wide variety of containers, including french fry boxes, pizza boxes and microwave popcorn bags.
- Avoid buying cosmetics and other personal care products with the phrase "fluoro" or "perfluoro" on the ingredient list. Among products that might contain PFCs are lotions, pressed powders, nail polish, and shaving cream.
** Legal Disclaimer. The conclusions and findings that appear on this page reflect EWG's research at the time of publication stated above. In light of evolving market conditions, subsequent product reformulations, and other factors, they may no longer be current. EWG makes no representations or warranties about any of the products that may appear on this page. EWG hereby disclaims all warranties with regard to any of the products that may appear on this page, including express, statutory, implied warranties of merchantability or fitness for a particular use.