View and Download our report here: Chromium-6 in U.S. Tap Water
Cancer-causing chemical found in 89 percent of cities sampled
Laboratory tests commissioned by Environmental Working Group have detected hexavalent chromium, the carcinogenic “Erin Brockovich chemical,” in tap water from 31 of 35 American cities. The highest levels were in Norman, Okla.; Honolulu, Hawaii; and Riverside, Calif. In all, water samples from 25 cities contained the toxic metal at concentrations above the safe maximum recently proposed by California regulators.
The National Toxicology Program has concluded that hexavalent chromium (also called chromium-6) in drinking water shows “clear evidence of carcinogenic activity” in laboratory animals, increasing the risk of gastrointestinal tumors. In September 2010, a draft toxicological review by the U.S. Environmental Protection Agency (EPA) similarly found that hexavalent chromium in tap water is “likely to be carcinogenic to humans.”
In 2009, California officials proposed setting a “public health goal” for hexavalent chromium in drinking water of 0.06 parts per billion (ppb) to reduce cancer risk. This was the first step toward establishing a statewide enforceable limit. Despite mounting evidence of its toxic effects, the EPA has not set a legal limit for hexavalent chromium in tap water nationally and does not require water utilities to test for it. In 25 cities where EWG’s testing detected chromium-6 — in the first publicly available national survey for the contaminant — it was found in concentrations exceeding California’s proposed maximum, in one case at a level more than 200 times higher.
At least 74 million Americans in 42 states drink chromium-polluted tap water, much of it likely in the cancer-causing hexavalent form. Given the scope of exposure and the magnitude of the potential risk, EWG believes the EPA should move expeditiously to establish a legal limit for chromium-6 and require public water suppliers to test for it.
View and Download our report here: Chromium-6 in U.S. Tap Water
View and Download our letter to the EPA here: EWG Tells EPA: Protect Public from Chromium-6 in Tap Water
Executive Summary
Chromium-6 – the Erin Brockovich Chemical – Is Widespread in U.S. Tap Water
Tests find cancer-causing chemical in 89 percent of cities sampled
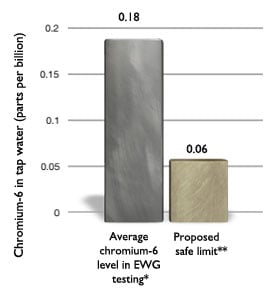
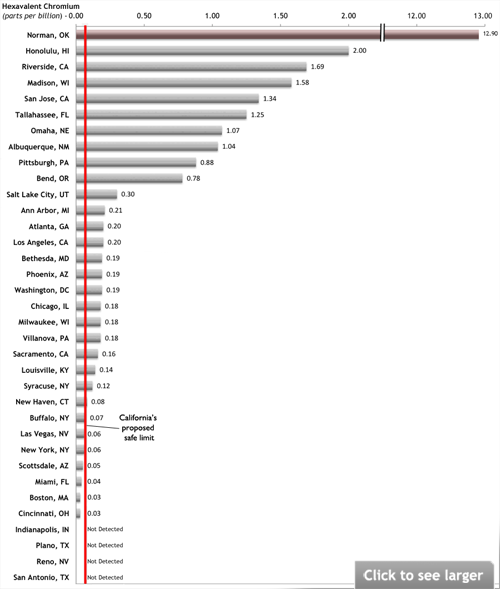
Chromium-6 in tap water of 35 cities averaged 3 times California's proposed safety goal
*Geometric average based on level of chromium-6 measured in 35 U.S. cities and a statistical estimate for the four cities where no chromium-6 was detected. The lowest level detectable by these tests is 0.02 ppb. For the purpose of calculating the nationwide average, the concentration of chromium-6 in these four cities was assumed to be 0.01 ppb, or half of the lowest detectable level. **"Proposed safe limit" is California EPA's proposed public health goal (OEHHA 2009). Source: EWG-commissioned testing for hexavalent chromium in tap water from 35 cities. |
Executive Summary
Tap water from 31 of 35 U.S. cities tested contains hexavalent chromium (or chromium-6), the carcinogenic “Erin Brockovich chemical,” according to laboratory tests commissioned by Environmental Working Group (EWG). The highest levels were detected in Norman, Okla.; Honolulu, Hawaii; and Riverside, Calif.
Despite mounting evidence of the contaminant’s toxic effects, including a U.S. Environmental Protection Agency (EPA) draft toxicological review that classifies it as “likely to be carcinogenic to humans” when consumed in drinking water, the agency has not set a legal limit for chromium-6 in tap water and does not require water utilities to test for it. Hexavalent chromium is commonly discharged from steel and pulp mills as well as metal-plating and leather-tanning facilities. It can also pollute water through erosion of soil and rock.
The National Toxicology Program has found that hexavalent chromium in drinking water shows clear evidence of carcinogenic activity in laboratory animals, increasing the risk of otherwise rare gastrointestinal tumors (NTP 2007, 2008). In response to this study and others, California officials last year proposed setting a public health goal for chromium-6 in drinking water of 0.06 parts per billion (ppb). This is the first step toward establishing a statewide enforceable limit (OEHHA 2009).
Levels of the carcinogen in 25 cities tested by EWG were higher than California’s proposed public health goal. Tap water from Norman, Okla. (population 90,000) contained more than 200 times California’s proposed safe limit.
Millions of Americans drink chromium-contaminated water
EWG’s investigation is the broadest publicly available survey of hexavalent chromium to date. The 31 cities with chromium-polluted tap water draw from utilities that collectively serve more than 26 million people. In California, the only state that requires testing for hexavalent chromium, water utilities have detected the compound in tap water supplied to more than 31 million people, according to an EWG analysis of data from the state water agency (EWG 2009).
Top five chromium-contaminated cities tested by EWG
| City | City Population | Hexavalent Chromium Contamination Level in Tap Water |
|---|---|---|
| Norman, Oklahoma | 89,952 | 12.9 ppb |
| Honolulu, Hawaii | 661,004 | 2.00 ppb |
| Riverside, California | 280,832 | 1.69 ppb |
| Madison, Wisconsin | 200,814 | 1.58 ppb |
| San Jose, California | 979,000 | 1.34 ppb |
EWG's tests provide a one-time snapshot of chromium-6 levels in 35 cities. But chromium pollution is a continuous, ongoing problem, as shown by the annual water quality reports that utilities must produce under federal law. Over the years, nearly all of the 35 cities tested by EWG regularly report finding chromium (in the form of total chromium) in their water despite using far less sensitive testing methods than those used by EWG.
The total number of Americans drinking tap water contaminated with this compound is likely far higher than is indicated by EWG's tests. At least 74 million people in nearly 7,000 communities drink tap water polluted with “total chromium,” which includes hexavalent and other forms of the metal, according to EWG’s 2009 analysis of water utility tests from 48,000 communities in 42 states (EWG 2009).
The EPA has set a legal limit in tap water for total chromium of 100 ppb to protect against “allergic dermatitis” (skin irritation or reactions). Measures of total chromium include the essential mineral trivalent chromium, which regulates glucose metabolism, as well as the cancer-causing hexavalent form. Preliminary EWG-commissioned water tests found that in most cases, the majority of the total chromium in water was in the hexavalent form, yet the EPA’s legal limit for total chromium is 1,700 times higher than California's proposed public health goal for hexavalent chromium. This disparity could indicate significant cancer risk for communities drinking chromium-tainted tap water.
The EPA’s new analysis of hexavalent chromium toxicity, released in draft form in September 2010 (EPA 2010a), cites significant cancer concerns linked to exposure to the contaminant in drinking water. It highlights health effects documented in animal studies, including anemia and damage to the gastrointestinal tract, lymph nodes and liver.
Industry deception delayed protections
The plight of the cancer-stricken residents of Hinkley, Calif., who in 1996 won a $333 million settlement from Pacific Gas and Electric Co. for contaminating their tap water with hexavalent chromium, was the basis of the 2000 movie “Erin Brockovich,” starring Julia Roberts.
Subsequently, a 2005 Wall Street Journal investigation and a separate EWG report based on court documents and depositions from a similar lawsuit in Kettleman City, Calif. revealed that PG&E had hired consultants to publish a fraudulent analysis of cancer mortality in Chinese villagers exposed to hexavalent chromium, in an attempt to disprove the link between the chemical and cancer. The study was published in the respected Journal of Occupational and Environmental Medicine, and scientists and regulators — including the EPA — cited the fraudulent article in research and safety assessments. The journal retracted the paper in 2006 in response to EWG’s request for corrective action.
California officials then conducted a rigorous re-assessment of the study data, finding a statistically significant increase in stomach cancer among the exposed. Their analysis is consistent with laboratory evidence from the National Toxicology Program and others showing that hexavalent chromium in tap water causes gastrointestinal tumors in multiple species.
Industry has sought for more than six years to delay state-mandated regulation of hexavalent chromium in tap water in California. Aerospace giant Honeywell International Inc. and others have stalled the adoption of the advisory public health goal by pressing for additional external scientific peer review. California’s Department of Public Health can neither set nor enforce a mandatory tap water standard for hexavalent chromium until the goal is finalized.
Recommendations
At least 74 million Americans in 42 states drink chromium-polluted tap water, much of it likely in the form of cancer-causing hexavalent chromium. Given the scope of exposure and the magnitude of the potential risk, the EPA should move expeditiously to establish a legal limit for the chemical in tap water and require water utilities to test for it.
The state of California must establish a strong standard for hexavalent chromium in tap water immediately. A truly health-protective hexavalent chromium regulation will reduce the cancer risk for Californians and serve as a model for the nation. With an enforceable standard already six years past the statutory deadline and the health of millions of Californians at stake, the state cannot move too quickly.
Study Findings
Carcinogenic Erin Brockovich Chemical Found in Tap Water Across the U.S.
Tests commissioned by the Environmental Working Group (EWG) detected carcinogenic hexavalent chromium in 31 of 35 tap water samples — 89 percent — collected in cities across the country. EWG targeted a mix of large cities and some smaller ones where testing by local water utilities had previously detected potentially significant amounts of “total chromium.” This less specific measurement includes trivalent chromium, an essential mineral that regulates glucose metabolism, as well the cancer-causing hexavalent form, also called chromium-6.
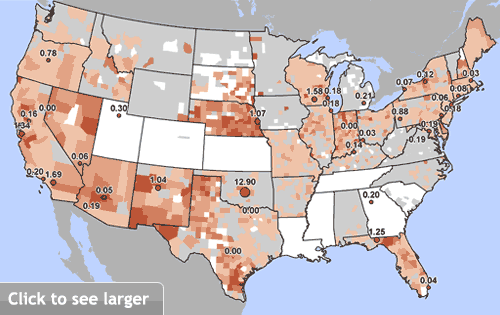
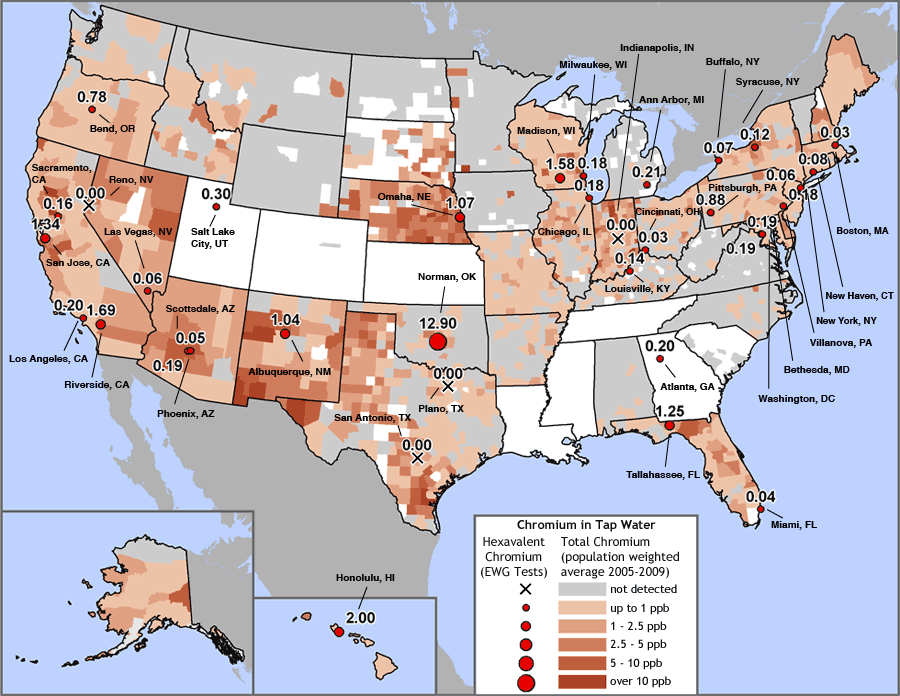
Chromium widely contaminates U.S. tap water
Red dots indicate EWG’s test sites and measured hexavalent chromium concentrations in parts per billion (ppb). Size of dot reflects the level found. Brown-shaded areas represent population-adjusted average concentrations of total chromium by county, calculated from EWG’s national tap water database (see Study Methodology).
Sources: EWG-commissioned testing for hexavalent chromium in tap water from 35 cities; EWG analysis of water utility testing data obtained from state water agencies (EWG 2009).
Hexavalent chromium (or chromium-6) gets into water supplies after being discharged from steel and pulp mills as well as metal-plating and leather-tanning facilities. It can also pollute water through erosion of soil and rock.
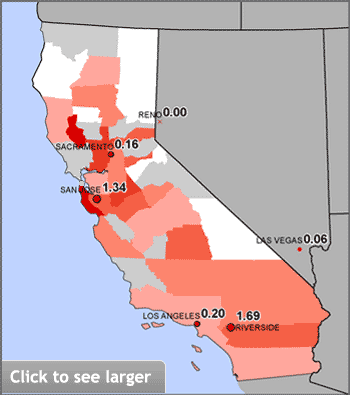
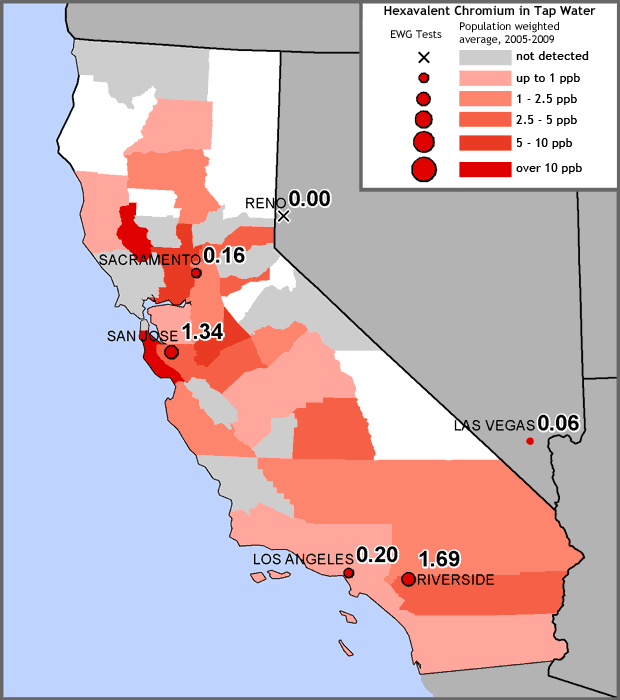
In California, the only state that requires water utilities to test for hexavalent chromium, the state’s Environmental Protection Agency (California EPA) has proposed a “public health goal,” or maximum safe concentration, of 0.06 parts per billion (ppb) in tap water to protect against excess cancer risk. However, the state’s current testing protocols are significantly less sensitive than those of the independent laboratory hired by EWG and may identify only the most extreme cases of contamination. Chromium-6 levels in tap water in all four California cities tested by EWG exceeded the proposed public health goal. (Once the goal is established, state regulators plan to embark on a rule-making process to set a legally enforceable upper limit.)
Chromium-6 is a common pollutant in California tap water
EWG measured concentrations of hexavalent chromium in four California cities — Los Angeles, Riverside, Sacramento and San Jose. Size of red dots reflects the level found. Colored areas reflect population-adjusted average concentrations of hexavalent chromium by county, as calculated from EWG’s tap water database (see Study Methodology). The state’s current testing protocols cannot detect chromium-6 in amounts lower than 1 ppb, more than 16 times higher than the proposed safe level.
Sources: EWG-commissioned testing for hexavalent chromium in tap water from four California cities; EWG analysis of water utility testing data obtained from state water agencies (EWG 2009).
Nationally, samples from 25 cities tested by EWG had levels of hexavalent chromium higher than the safe limit proposed in California.
Chromium-6 levels in 25 cities’ tap water exceed safe limit proposed by California officials*
Source: EWG-commissioned testing for hexavalent chromium in tap water from 35 cities.
*Proposed safe limit is California EPA's proposed public health goal (OEHHA 2009).
For total chromium, the US Environmental Protection Agency has set a legal limit of 100 ppb in tap water to protect against “allergic dermatitis” (skin irritation or reactions). California’s legal limit for total chromium is half that — 50 ppb.
EWG’s analysis of California’s tap water testing data indicates that chromium-6 constitutes more than half of the total chromium found in most water supplies, a finding further supported by initial data from EWG’s nationwide survey. A proprietary 2004 study by the Water Research Foundation for its paying members, including water utilities, found that hexavalent chromium contamination of tap water was more common for systems using groundwater wells than for those drawing surface water (AWWARF 2004). The EPA’s 100 ppb legal limit for total chromium is more than 1,600 times higher than the California’s proposed public health goal for hexavalent chromium. This could mean that communities with higher concentrations of total chromium face a cancer risk well above the levels typically considered safe.
Chromium widely contaminates U.S. tap water
Red dots indicate EWG’s test sites and measured hexavalent chromium concentrations in parts per billion (ppb). Size of dot reflects the level found. Brown-shaded areas represent population-adjusted average concentrations of total chromium by county, calculated from EWG’s national tap water database (see Study Methodology).
Sources: EWG-commissioned testing for hexavalent chromium in tap water from 35 cities; EWG analysis of water utility testing data obtained from state water agencies (EWG 2009).
Chromium widely contaminates U.S. tap water
Red dots indicate EWG’s test sites and measured hexavalent chromium concentrations in parts per billion (ppb). Size of dot reflects the level found. Brown-shaded areas represent population-adjusted average concentrations of total chromium by county, calculated from EWG’s national tap water database (see Study Methodology).
Sources: EWG-commissioned testing for hexavalent chromium in tap water from 35 cities; EWG analysis of water utility testing data obtained from state water agencies (EWG 2009).
Chromium-6 is a common pollutant in California tap water
EWG measured concentrations of hexavalent chromium in four California cities — Los Angeles, Riverside, Sacramento and San Jose. Size of red dots reflects the level found. Pink-shaded areas reflect population-adjusted average concentrations of hexavalent chromium by county, as calculated from EWG’s tap water database (see Study Methodology). The state’s current testing protocols cannot detect chromium-6 in amounts lower than 1 ppb, more than 16 times higher than the proposed safe level.
Sources: EWG-commissioned testing for hexavalent chromium in tap water from four California cities; EWG analysis of water utility testing data obtained from state water agencies (EWG 2009).
Chromium-6 levels in 25 cities’ tap water exceed safe limit proposed by California officials*
Source: EWG-commissioned testing for hexavalent chromium in tap water from 35 cities.
*Proposed safe limit is California EPA's proposed public health goal (OEHHA 2009).
Industry Tactics
Industry falsified key study of “Erin Brockovich chemical”
Chromium is a naturally occurring metal used in steel manufacturing, leather tanning, welding and the production of dyes, pigments and alloys. It is often used to plate metal surfaces and is a major component of pesticides used in pressure-treated lumber for outdoor decks, play sets and other structures (one form was banned in 2005). Chromium was also widely used as an anti-corrosive agent in industrial cooling towers until the federal government banned the practice in 1990 (EPA 2000). It is an essential component in making stainless steel, its most common use, and super-alloys (USGS 2010).
The toxic form of chromium is not regulated in tap water
Chromium has multiple forms, and the two most common have dramatically different consequences for human health. Trivalent chromium (chromium-3) is a nutrient essential to sugar and lipid metabolism, but hexavalent chromium (chromium-6) is a dangerous toxin. Since 1990, international health authorities have identified it as a known human carcinogen when inhaled (IARC 1990), and a growing body of evidence has linked hexavalent chromium in drinking water to stomach and gastrointestinal cancers.
In 1992, the EPA set the legal limit in tap water for total chromium — a mixture of hexavalent and trivalent chromium — at 100 ppb to protect against skin reactions known as “allergic dermatitis” (EPA 2010b). However, a safety standard that lumps levels of a toxic carcinogen with a nutrient necessary for health is like grouping arsenic and vitamin C.
Recent California Department of Public Health tests of drinking water detected hexavalent chromium in 2,208 of more than 7,000 water sources (CDPH 2009). A review of EWG’s tap water quality database indicates that more than 74 million Americans may be exposed to total chromium through tap water, and more than 13.7 million Californians may be exposed to hexavalent chromium (EWG 2009).
New evidence overturns claims that chromium-6 is harmless
Various conditions can cause trivalent chromium to change to hexavalent chromium and vice versa. The widely used tap water disinfectant chlorine, for instance, can cause trivalent to become hexavalent (Lai 2006). Highly acidic conditions can cause hexavalent to become trivalent. For years, scientists assumed that all hexavalent chromium was converted to trivalent by the stomach’s acidic environment, rendering it harmless.
It is now clear, however, that some of this toxic chemical can pass through the stomach unchanged and penetrate tissues and organs throughout the body (Costa 1997). Studies in both animals and people show that exposure to hexavalent chromium via drinking water leads to elevated chromium levels in tissues, particularly the gastrointestinal tract, blood, liver, kidneys and spleen, and in increased toxicity (Kerger 1996; Finley 1997; Anderson 2002; NTP 2008; EPA 2010a).
Industry deceit covered up cancer connection
Research on the effects of chromium-6 in drinking water has focused on increased cancer risk. More than 20 years ago, researchers found an increased risk of stomach cancer and a “significant excess of overall cancer mortality” among villagers in China’s Liaoning Province whose drinking water had been polluted by a chromium ore processing facility (Zhang 1987).
This research should have triggered a flurry of scientific and regulatory scrutiny, but the study was published in a Chinese-language medical journal, making it largely inaccessible to U.S. researchers and regulators. Ten years later, in April 1997, the Journal of Occupational and Environmental Medicine (JOEM) published a paper, purportedly by the same Chinese research team, that reversed the earlier conclusion. It said that the data from Liaoning Province “do not indicate an association of cancer mortality with exposure to [hexavalent chromium]-contaminated groundwater” (Zhang 1997).
Investigations by EWG and the Wall Street Journal (EWG 2005) revealed that ChemRisk, a consulting firm hired by Pacific Gas & Electric Co. (PG&E) to fight the Erin Brockovich lawsuit over contamination in Hinkley, Calif., had distorted data from the Chinese study and placed the falsified paper in a respected scientific journal in order to reverse the original conclusion linking hexavalent chromium to stomach cancer.
Exposé outed corrupt consultant
EWG’s review of documents and depositions from a Kettleman City, Calif. lawsuit against PG&E revealed that ChemRisk’s employees — with the knowledge of PG&E’s attorneys — had conducted their own analysis of the original Chinese data in 1995-97, deliberately excluding reports of cancer cases in the province that pointed to an association with hexavalent chromium. They then wrote and submitted their paper for publication without disclosing that they worked for ChemRisk or that PG&E had paid for the new “study.”
Kettleman City, like Hinkley, is home to a PG&E station that pumps natural gas from a Texas pipeline to California customers. Both facilities used hexavalent chromium to cool the natural gas and then dumped it into unlined ponds that allowed the contaminant to leach into groundwater.
In the Brockovich lawsuit, residents of Hinkley sued PG&E for polluting their tap water with hexavalent chromium — the basis for the Julia Roberts film released in 2000. PG&E paid $333 million to settle the Hinkley case before the falsified paper was published, but scientists and regulators — including the EPA — subsequently cited the paper in research and safety assessments. In response to EWG's request for corrective action (EWG 2006), the journal retracted the paper in 2006, citing in particular the fact that “financial and intellectual input to the paper by outside parties was not disclosed” (Brandt-Rauf 2006). Also in 2006, PG&E settled with the Kettleman City victims of chromium-6 contamination for $335 million.
As part of its toxicological review, the California Environmental Protection Agency’s (California EPA) Office of Environmental Health Hazard Assessment (OEHHA), charged with setting a public health goal for the contaminant in tap water, conducted a rigorous re-analysis of the Chinese data. That work once again demonstrated a statistically significant increase in stomach cancer among the hexavalent chromium-exposed villagers compared to Liaoning Province’s overall population (Beaumont 2008).
Laboratory studies bolster cancer link
Animal studies have provided additional evidence linking hexavalent chromium to cancer. A study by federal toxicologists on rats and mice revealed statistically significant, dose-related increases in tumors of the duodenum and small intestine in mice, and statistically significant increases in tumors of the oral cavity in rats (NTP 2008). Based on these data, the National Toxicology Program’s (NTP) Board of Scientific Counselors concluded that hexavalent chromium in drinking water shows clear evidence of carcinogenic activity (NTP 2007).
These results agree with those of an earlier study that was marred by a number of limitations, including the outbreak of a viral infection in the mice under study (Borneff 1968). Nevertheless, a thorough statistical analysis of these data that accounted for the limitations still found a significant increase in stomach tumors (OEHHA 2009).
The NTP findings led the US EPA to list hexavalent chromium as a priority for evaluation under its Integrated Risk Information System (IRIS), which last reviewed the health concerns associated with this contaminant in 1998. In September 2010, the agency released a draft toxicological review, concluding that chromium-6 in drinking water is “likely to be carcinogenic to humans” (EPA 2010a). Unfortunately, the EPA has also cited its ongoing investigation as a reason to delay adopting a more health-protective federal limit for chromium in tap water (EPA 2009).
In contrast, California has moved ahead. California EPA scientists drew a clear conclusion: “The findings of available human, animal, genotoxic, and toxicokinetic studies all indicate that hexavalent chromium is a possible human carcinogen by the oral route” (OEHHA 2009). Dr. R. Gwiazda, a reviewer of the draft public health goal for chromium-6 in tap water, summed it up best: “Overall, the document convincingly demonstrates that indeed there is a relationship between exposure to [hexavalent chromium] via the oral route and the development of cancer in the gastrointestinal tract” (Gwiazda 2008).
Some people are especially vulnerable
Some individuals may be especially susceptible to the carcinogenic effects of chromium-6. Specifically, people with less acidic stomachs appear to have limited ability to convert hexavalent chromium to trivalent chromium, exposing them to higher levels of the toxic form and putting them at greater risk.
A low-acid stomach can be caused by several widely used medications, such as antacids and proton pump inhibitors used to treat common disorders including gastroesophageal reflux disease, peptic ulcer disease and chronic gastritis. Other conditions that can inhibit stomach acid production include pernicious anemia, pancreatic tumors, infection with Helicobacter pylori (a common bacterium linked to ulcers), mucolipidosis type IV and some autoimmune diseases. People with pernicious anemia have also been found to absorb hexavalent chromium more readily (Donaldson 1966).
Fetuses, infants and children also have higher sensitivity to carcinogenic chemicals. According to the National Academy of Sciences (NAS), children’s developing organ systems are more vulnerable to damage from chemical exposures, and children are less able than adults to detoxify and excrete chemicals (NAS 1993). A recent evaluation by US EPA scientists in response to the agency’s 2005 revised Cancer Guidelines noted that hexavalent chromium causes germ cell mutations and DNA deletions in developing embryos, indicating a need for age-dependent adjustment factors for risk assessments to account for the toxin’s increased damage in developing bodies (McCarroll 2010).
Chronic exposure to hexavalent chromium in tap water is likely to raise everyone’s risk of cancer, but the young and the medically impaired may be especially vulnerable. These susceptible subpopulations deserve special protections.
Government Failings
EPA slow to set drinking water limits for chromium-6
Despite growing recognition of hexavalent chromium’s carcinogenic potential, including EPA’s draft designation of it as a likely human carcinogen, the agency has taken no action to limit levels of this toxic compound in drinking water. The agency has left in place an inadequate standard for total chromium, set nearly 20 years ago, that does not distinguish between toxic hexavalent and nutritionally essential trivalent chromium and cites “allergic dermatitis” as the only relevant health concern.
The EPA has reviewed its standard for total chromium twice since setting it in 1992. In 2003, the agency determined that even though new research on chromium-6 indicated cause for concern, information gaps prevented establishment of a more protective standard (EPA 2003). Six years later, the EPA again delayed action on a stricter standard, this time because it had initiated an evaluation of hexavalent chromium via its Integrated Risk Information System (IRIS) (EPA 2009). The draft toxicological review released in September as part of this process identified exposure to hexavalent chromium in drinking water as likely to cause cancer to humans, and cited animal studies linking it to a variety of other health effects, including anemia and damage to the gastrointestinal tract, lymph nodes and liver (EPA 2010a).
Drinking water standards are drastically out-of-date
The EPA’s inaction is but one example of the agency’s lack of resolve in protecting Americans’ tap water. The agency has not set a new, enforceable drinking water standard for any contaminant since 2001, even though the Safe Drinking Water Act requires the EPA to assess the need for standards for at least five new chemicals every five years. Three-fourths of the current standards, including for total chromium, were set in 1991 and 1992 and have not been updated since.
Since 1996, the EPA has reviewed data on toxicity and water pollution for 138 chemicals, but in every case it declined to set a safety standard. EWG's analysis of its tap water quality database showed that collectively these chemicals pollute drinking water used by more than 111 million Americans (EWG 2009).
The framework under which the EPA sets drinking water standards is outdated. For example, the agency is not required to set maximum legal limits for contaminants at levels that protect the health of children or to consider the heightened vulnerability of the fetus and newborns (Donohue 2002).
In addition, the EPA sets maximum legal limits for contaminants as if people are exposed to just one at a time. That’s not the reality — research shows that people carry hundreds of chemicals in their bodies at any given time. A growing number of studies also show that the risks add up when people are exposed to multiple chemicals that can act in tandem to cause harm — and that total risk can be greater than the sum of the parts (NRC 2008).
At long last, signs of progress
For the 114 contaminants that the EPA does regulate, EWG’s drinking water quality analysis found that water suppliers achieved 92 percent compliance with mandatory health standards, demonstrating that utilities can and do meet enforceable limits when they exist (EWG 2009). However, the EPA’s failure to develop meaningful standards for hexavalent chromium and scores of other contaminants leaves the public at risk.
Recently the federal government has begun to focus a critical eye on hexavalent chromium and other water contaminants. When EPA Administrator Lisa Jackson took office, she announced that protecting America’s drinking water would be one of seven agency priorities. In keeping with this goal, the EPA has announced plans to set a legal limit for perchlorate in tap water, which would make it the first new chemical to be regulated in drinking water in a decade. Meanwhile, the Toxic Chemicals Safety Act (H.R. 5820), introduced in the House of Representatives this summer, specifically lists hexavalent chromium as a priority chemical for safety evaluation.
EWG recommends that the EPA set a legal limit for hexavalent chromium in drinking water as quickly as possible and require all water utilities to test for it. The EPA can speed the process by streamlining the IRIS assessment. We hope that Administrator Jackson’s leadership on this critical issue will reduce cancer risk for all Americans.
Progress in California
California Moving Slowly in the Face of Industry Resistance
State law required California to adopt a drinking water standard for hexavalent chromium, the “Erin Brockovich chemical,” by Jan. 1, 2004. But with a legislature that regularly disregards its constitutional deadline for adopting a state budget, it is hardly surprising that state agencies now lag more than six years behind in protecting residents from this cancer-causing contaminant.
In August 2009, the Office of Environmental Health Hazard Assessment (OEHHA), part of California’s Environmental Protection Agency, completed the first step in the process, releasing a draft “public health goal” for chromium-6 in tap water (OEHHA 2009). The agency proposed a goal of 0.06 parts per billion (ppb) to limit the increased lifetime cancer risk to one additional case of cancer for every million people chronically exposed at this level through drinking water.
The California EPA, however, did not take into account the special sensitivity of fetuses and infants, as recommended recently by federal EPA scientists (McCarroll 2010), or of people with common medical conditions that may increase uptake of hexavalent chromium. An exposure limit of 0.06 ppb may not adequately protect the health of many Californians.
Industry, meanwhile, has pushed back. Honeywell International, Inc., along with the Association of California Water Agencies, has filed requests for an additional external scientific peer review of the draft document. (In 2003, a federal judge in Newark, N.J. ordered Honeywell, a producer of aerospace systems, engineering services and consumer products, to carry out an estimated $400 million cleanup of chromium waste along Jersey City’s waterfront, citing “a substantial risk of imminent damage to public health and safety and imminent and severe damage to the environment.”) The American Chemistry Council, an industry trade group, sought to rewrite the charge of the second peer review committee and influence the composition of the group (ACC 2010), all in an effort to weaken the proposed public health goal.
Four of the five independent scientists taking part in this additional, industry-instigated review process, now complete, expressed strong support for the proposed public health goal for hexavalent chromium (OEHHA 2010).
Concentrations of chromium-6 in tap water signal concern
In California, the only state to require tap water tests for hexavalent chromium, current water pollution levels are a cause for concern. The chemical was detected in 2,208 out of more than 7,000 tap water systems analyzed as of 2008 (CDPH 2009). These tests could only detect hexavalent chromium down to 1 ppb, more than 16 times higher than the state’s proposed public health goal. About 10 percent of the samples had levels of 5 ppb or higher.
EWG’s tap water quality database, including more recent test information, shows that 13.7 million Californians could be drinking water contaminated with at least 1 ppb of hexavalent chromium (EWG 2009). With a more sensitive test, hexavalent chromium would be detected in far more water systems.
Currently, California’s tap water standard for total chromium is 50 ppb, half the federal standard. Both the federal and state standards combine hexavalent chromium and the essential nutrient trivalent chromium, and are more than 800 and 1,600 times higher, respectively, than the proposed California public health goal for chromium-6. The fact that these regulations lump a cancer-causing contaminant with an essential nutrient underscores the need for reform of water standards.
Inching towards a tap water standard
The California Safe Drinking Water Act of 1996 requires the California EPA to perform risk assessments and adopt goals for contaminants in drinking water based on public health considerations alone. These goals do not have the force of regulation and represent only the first step in creating a mandatory standard.
Once the California EPA has finalized its public health goal for hexavalent chromium, the California Department of Public Health (CDPH) must establish a state drinking water standard known as a Maximum Contaminant Level. These standards take economic factors and technical challenges into account and should be as close as feasible to the corresponding public health goal.
EWG urges the California EPA to promptly finalize its public health goal for hexavalent chromium and calls on the CDPH to take immediate action to establish a sound regulatory standard. Regulation of this extremely common contaminant is already six years overdue.
Acknowledgments
Principal Author: Rebecca Sutton, PhD
Editors: Jane Houlihan, Renee Sharp & Nils Bruzelius
Databases & Mapping: Chris Campbell & Sean Gray
Web Design: Dean Clark
This report was made possible by the support of the John Merck Fund, the Johnson Family Foundation, the Park Foundation and the Turner Foundation.
EWG thanks Erin Brockovich and Bob Bowcock for their continued efforts to protect public health, and Max Costa (New York University School of Medicine) for his review of our report.
Interns Jacob Booher, Marisa Evanouski, Samara Geller and Kimi Schell made significant contributions to this research. We also thank EWG contacts nationwide who made this study possible by volunteering to collect water samples for analysis.
Frequently Asked Questions
- What is hexavalent chromium?
- How does it get into tap water?
- Why is it a problem?
- How can I find out if my tap water has hexavalent chromium in it?
- My tap water has high levels. What should I do?
- Can I test my own tap water?
- Besides drinking water, how else might I be exposed?
- Are some people more vulnerable to the effects?
- What other chemicals in my tap water should I be concerned about?
- What is EPA doing to promote safe drinking water?
What is hexavalent chromium?
Hexavalent chromium (or chromium-6) is a highly toxic form of the naturally occurring metal chromium. It is a well-known human carcinogen when inhaled, and recent evidence indicates it can cause stomach or gastrointestinal cancer when ingested in drinking water. A different form of this metal, trivalent chromium, is an essential nutrient.
Exposure to hexavalent chromium commonly occurs through consuming contaminated water or food, as well as by breathing contaminated air in the workplace, especially for those working in metallurgy or leather-tanning facilities. Contaminated soil particles may also be a source of exposure via ingestion or inhalation. Widespread industrial use has led to detections of hexavalent chromium in two-thirds of current or former Superfund sites.
How does it get into tap water?
Hexavalent chromium can enter water through industrial contamination from manufacturing facilities, including electroplating factories, leather tanneries and textile manufacturing facilities, or from disposal of fluids used in cooling towers before 1990. It also occurs naturally in some minerals. The commonly used tap water disinfectant chlorine can transform trivalent chromium into toxic hexavalent chromium.
Why is it a problem?
Exposure in tap water has been linked to cancers of the stomach and gastrointestinal tract in both animals and people. California’s Environmental Protection Agency has released a draft public health goal based on the conclusion that levels of chromium-6 greater than 0.06 parts per billion (ppb) in tap water may increase cancer risk.
Some people may be especially susceptible to the carcinogenic effects of chromium-6. Fetuses, infants, and children have higher sensitivity to carcinogenic chemicals. In addition, people with less acidic stomachs appear to have a limited ability to convert hexavalent chromium to trivalent chromium (chromium-3), exposing them to higher levels of the toxic form and putting them at greater risk. Using common antacids and proton pump inhibitors can reduce stomach acidity. Other conditions that can inhibit stomach acid production include infection with Helicobacter pylori (a common bacterium linked to ulcers), pernicious anemia, pancreatic tumors, mucolipidosis type IV and some autoimmune diseases.
How can I find out if my tap water has hexavalent chromium in it?
California requires water utilities to test and report levels of chromium-6 in their water. For Californians, this is a good way to see if this contaminant is a concern in your area. Unfortunately, these tests only measure levels at or above 1 ppb, more than 16 times higher than the suggested public health goal of 0.06 ppb. Of the 438 community water sources in California that have provided test data to EWG, 223 detected levels over 1 ppb and 93 detected levels over 5 ppb. This means more than 13.7 million Californians drink tap water contaminated with hexavalent chromium.
Elsewhere, water utilities only test and report levels of total chromium — which includes both the toxic form and the essential nutrient chromium-3. These tests only measure levels at or above 10 ppb, more than 160 times higher than California’s proposed public health goal. If your tap water has detectable levels of total chromium, it’s quite possible that it has levels of hexavalent chromium that violate California’s suggested public health goal. The ratio of chromium-3 to chromium-6 varies for different water supplies, so it is difficult to estimate how much of each form might be in your water.
Contact your local water utility or check EWG’s tap water database to learn if chromium has been detected in your tap water.
My tap water has high levels. What should I do?
If your tap water contains high levels, your best bet is to install a reverse osmosis water filter certified to remove this contaminant. Reverse osmosis filters, especially when combined with superior carbon filter technology, are best for ridding tap water of the largest number of contaminants possible. EWG provides a list of reverse osmosis water filters certified to remove hexavalent chromium and available for purchase on Amazon.
See EWG’s water filter buying guide for more information on how to select a water filter.
While drinking bottled water might seem like a good way to avoid exposing yourself to hexavalent chromium in tap water, there is no guarantee that bottled water has lower concentrations of this contaminant. If you drink bottled water, choose brands that provide water quality information indicating their water has levels of chromium-6 below 0.06 ppb or that use reverse osmosis filtration to treat their water.
Because infants can be especially sensitive to carcinogenic chemicals, it is particularly important to use safer water when preparing infant formula. Water treated with a reverse osmosis home filter will contain fewer contaminants and be safer for babies.
Can I test my own tap water for chromium-6?
Most commercial water quality laboratories do not offer this test.
Besides drinking water, how else can I be exposed?
Other sources of exposure to hexavalent chromium include contaminated food and contaminated workplace air, especially for those working in metallurgy or leather-tanning facilities. Contaminated soil particles may also be a source of exposure via ingestion or inhalation. Widespread industrial use has led to detections of chromium-6 in two-thirds of current or former Superfund sites.
Are some people more vulnerable to the effects?
Yes. Fetuses, infants, and children have a higher sensitivity to carcinogenic chemicals. Their developing organ systems are more susceptible to damage from chemical exposures, and less able to detoxify and excrete chemicals.
In addition, people with less acidic stomachs appear to have a limited ability to convert chromium-6 to chromium-3, exposing them to higher levels of the toxic form and putting them at greater risk. Using common antacids and proton pump inhibitors can reduce stomach acidity. Other conditions that can inhibit stomach acid production include infection with Helicobacter pylori (a common bacterium linked to ulcers), pernicious anemia, pancreatic tumors, mucolipidosis type IV and some autoimmune diseases.
What other chemicals in my tap water should I be concerned about?
Check out EWG’s tap water database for an in-depth look at water contaminants, including drinking water quality information for 48,000 communities in 45 states and the District of Columbia.
What is EPA doing to promote safe drinking water?
Not enough. In the case of hexavalent chromium, the EPA has taken no action to specifically limit levels of this toxic compound in drinking water. The agency has left in place an inadequate standard for total chromium, set nearly 20 years ago, that does not distinguish between toxic hexavalent and nutritionally essential trivalent chromium and cites “allergic dermatitis” as the only relevant health concern. The agency has not set a new, enforceable drinking water standard for any contaminant since 2001.
Recently the federal government has begun to focus a critical eye on chromium-6 and other water contaminants. EWG recommends that the EPA set a legal limit for hexavalent chromium in drinking water as quickly as possible and require water utility testing to assess exposures nationwide.
Tips for Consumers
Drinking plenty of good, clean water is important for a healthy body. Read EWG researchers’ top tips to learn how to stay hydrated while reducing your exposures to common drinking water pollutants.
Chromium-6 in tap water - filter it out at home.
The best way to remove chromium-6 from tap water at home is with a reverse osmosis filter. These filtration systems can be expensive, but with proper maintenance they can last at least a decade, bringing the cost down to as little as $5 per month. There's no legal limit for chromium-6 in either tap water or bottled water, so there’s no guarantee that either one is free of this contaminant.
Tap water - learn what's in it.
Tap water suppliers publish all their water quality tests. Bottled water companies don’t. Read your annual tap water quality report. Look up your city’s water in EWG’s National Tap Water Atlas (www.ewg.org/tap-water). (Private well? Get it tested.)
Filtered tap water - drink it, cook with it.
- Choose a filter certified to remove contaminants found in your water: www.ewg.org/tap-water/getawaterfilter. Effectiveness varies - read the fine print.
- Carbon filters (pitcher or tap-mounted) are affordable and reduce many common water contaminants, such as lead and byproducts of the disinfection process used to treat municipal tap water.
- If you can afford it, install a reverse osmosis filter to remove contaminants that carbon filters can’t eliminate, such as chromium-6, arsenic and perchlorate (rocket fuel).
Filters - change them.
Change your water filters on time. Old filters aren’t safe – they harbor bacteria and let contaminants through.
Bottled water - drink filtered tap water instead.
You can read the bottle label and still not know whether the water is pure or just processed tap water. EWG found 38 contaminants in 10 popular brands.
On the go - carry water in safe containers.
Hard plastic bottles (#7 plastic) can leach a harmful plastics chemical called bisphenol-A (BPA) into water. Carry stainless steel or other BPA-free bottles. Don’t reuse bottled water bottles. The plastic can harbor bacteria and break down to release plastics chemicals.
While pregnant - stay hydrated with safe water.
It’s especially important for women to drink plenty of water during pregnancy. Follow all the tips above and take your doctor’s advice on how much to drink.
Infants - use safe water for formula.
Use filtered tap water for your baby’s formula. If your water is not fluoridated, you can use a carbon filter. If it is, use a reverse osmosis filter to remove the fluoride, because fluoridated water can damage an infant’s developing teeth. If you choose bottled water for your infant, make sure it’s fluoride-free. Learn more at www.ewg.org/babysafe.
Breathe easy - use a whole house water filter.
For extra protection, a whole house carbon filter will remove contaminants from steamy vapors you and your family inhale while showering and washing dishes. Effectiveness varies widely – call the manufacturer for details.